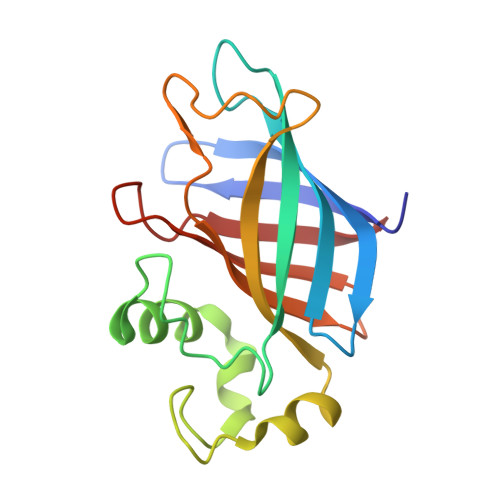
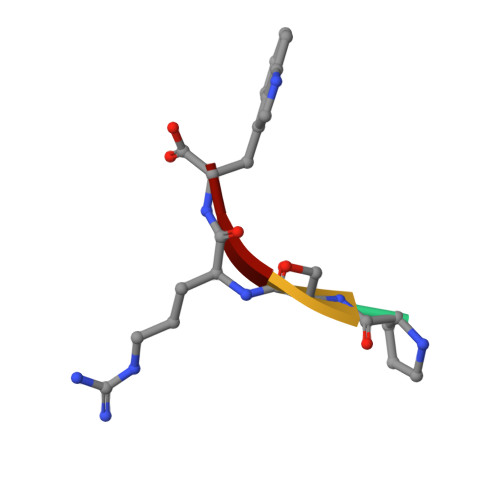
Molecular basis of GID4-mediated recognition of degrons for the Pro/N-end rule pathway.
Dong, C., Zhang, H., Li, L., Tempel, W., Loppnau, P., Min, J.(2018) Nat Chem Biol 14: 466-473
- PubMed: 29632410
- DOI: https://doi.org/10.1038/s41589-018-0036-1
- Primary Citation of Related Structures:
6CCR, 6CCT, 6CCU, 6CD8, 6CD9, 6CDC, 6CDG - PubMed Abstract:
The N-end rule pathway senses the N-terminal destabilizing residues of degradation substrates for the ubiquitin-proteasome system, whose integrity shields against various human syndromes including cancer and cardiovascular diseases. GID4, a subunit of the ubiquitin ligase GID complex, has been recently identified as the N-recognin of the new branch of the N-end rule pathway responsible for recognizing substrates bearing N-terminal proline residues (Pro/N-degrons). However, the molecular mechanism of GID4-mediated Pro/N-degron recognition remains largely unexplored. Here, we report the first crystal structures of human GID4 alone and in complex with various Pro/N-degrons. Our complex crystal structures, together with biophysical analyses, delineate the GID4-mediated Pro/N-degron recognition mechanism and substrate selection criteria for the Pro/N-end rule pathway. These mechanistic data on the Pro/N-recognin activity of GID4 will serve as a foundation to facilitate the identification of authentic physiological substrates as well as the development of inhibitors of therapeutic values for the Pro/N-end rule pathway.
Organizational Affiliation:
Structural Genomics Consortium, University of Toronto, Toronto, ON, Canada.