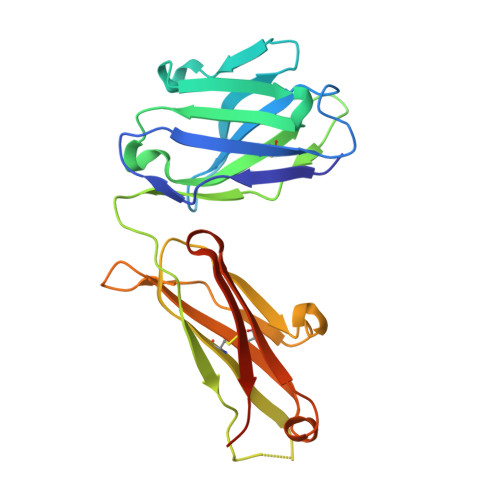
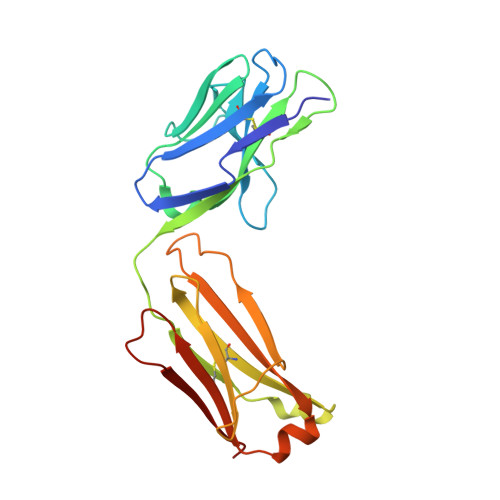
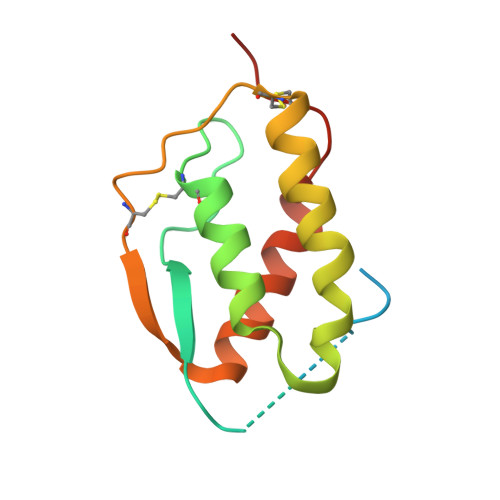
The mechanism of GM-CSF inhibition by human GM-CSF auto-antibodies suggests novel therapeutic opportunities.
Dhagat, U., Hercus, T.R., Broughton, S.E., Nero, T.L., Cheung Tung Shing, K.S., Barry, E.F., Thomson, C.A., Bryson, S., Pai, E.F., McClure, B.J., Schrader, J.W., Lopez, A.F., Parker, M.W.(2018) MAbs 10: 1018-1029
- PubMed: 29969365
- DOI: https://doi.org/10.1080/19420862.2018.1494107
- Primary Citation of Related Structures:
6BFQ, 6BFS - PubMed Abstract:
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a hematopoietic growth factor that can stimulate a variety of cells, but its overexpression leads to excessive production and activation of granulocytes and macrophages with many pathogenic effects. This cytokine is a therapeutic target in inflammatory diseases, and several anti-GM-CSF antibodies have advanced to Phase 2 clinical trials in patients with such diseases, e.g., rheumatoid arthritis. GM-CSF is also an essential factor in preventing pulmonary alveolar proteinosis (PAP), a disease associated with GM-CSF malfunction arising most typically through the presence of GM-CSF neutralizing auto-antibodies. Understanding the mechanism of action for neutralizing antibodies that target GM-CSF is important for improving their specificity and affinity as therapeutics and, conversely, in devising strategies to reduce the effects of GM-CSF auto-antibodies in PAP. We have solved the crystal structures of human GM-CSF bound to antigen-binding fragments of two neutralizing antibodies, the human auto-antibody F1 and the mouse monoclonal antibody 4D4. Coordinates and structure factors of the crystal structures of the GM-CSF:F1 Fab and the GM-CSF:4D4 Fab complexes have been deposited in the RCSB Protein Data Bank under the accession numbers 6BFQ and 6BFS, respectively. The structures show that these antibodies bind to mutually exclusive epitopes on GM-CSF; however, both prevent the cytokine from interacting with its alpha receptor subunit and hence prevent receptor activation. Importantly, identification of the F1 epitope together with functional analyses highlighted modifications to GM-CSF that would abolish auto-antibody recognition whilst retaining GM-CSF function. These results provide a framework for developing novel GM-CSF molecules for PAP treatment and for optimizing current anti-GM-CSF antibodies for use in treating inflammatory disorders.
Organizational Affiliation:
a St. Vincent's Institute of Medical Research , Australian Cancer Research Foundation Rational Drug Discovery Centre , Fitzroy , Victoria , Australia.