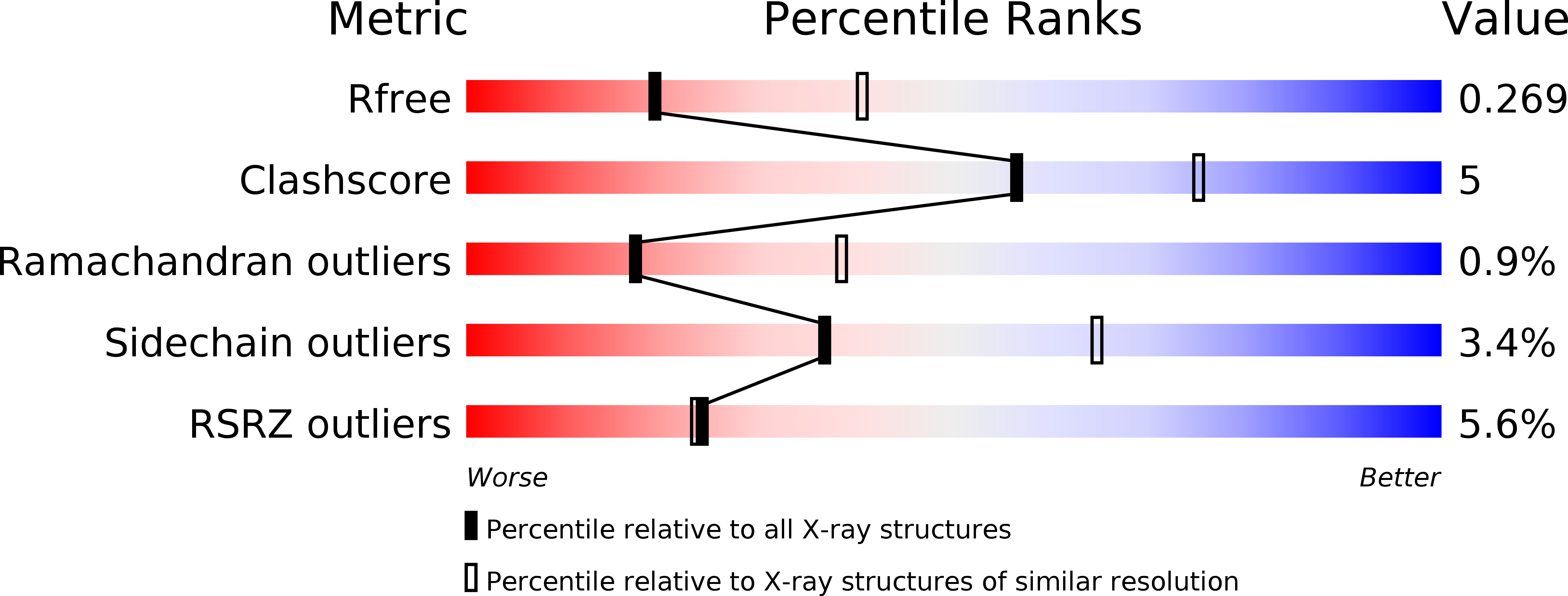
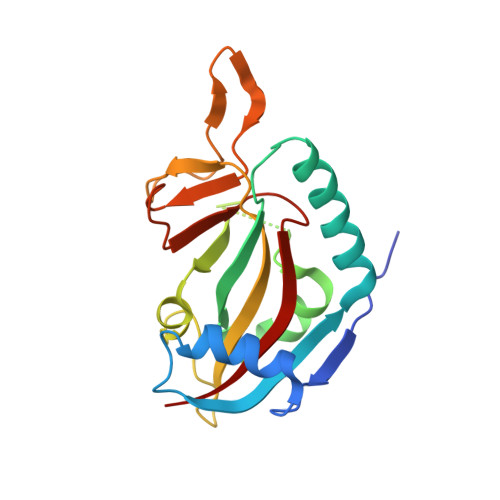
Design and synthesis of potent inhibitors of the mono(ADP-ribosyl)transferase, PARP14.
Upton, K., Meyers, M., Thorsell, A.G., Karlberg, T., Holechek, J., Lease, R., Schey, G., Wolf, E., Lucente, A., Schuler, H., Ferraris, D.(2017) Bioorg Med Chem Lett 27: 2907-2911
- PubMed: 28495083
- DOI: https://doi.org/10.1016/j.bmcl.2017.04.089
- Primary Citation of Related Structures:
5NQE - PubMed Abstract:
A series of (Z)-4-(3-carbamoylphenylamino)-4-oxobut-2-enyl amides were synthesized and tested for their ability to inhibit the mono-(ADP-ribosyl)transferase, PARP14 (a.k.a. BAL-2; ARTD-8). Two synthetic routes were established for this series and several compounds were identified as sub-micromolar inhibitors of PARP14, the most potent of which was compound 4t, IC 50 =160nM. Furthermore, profiling other members of this series identified compounds with >20-fold selectivity over PARP5a/TNKS1, and modest selectivity over PARP10, a closely related mono-(ADP-ribosyl)transferase.
Organizational Affiliation:
McDaniel College, 2 College Hill, Westminster, MD 21157, United States.