Discovery and Characterization of 2-Acylaminoimidazole Microsomal Prostaglandin E Synthase-1 Inhibitors.
Schiffler, M.A., Antonysamy, S., Bhattachar, S.N., Campanale, K.M., Chandrasekhar, S., Condon, B., Desai, P.V., Fisher, M.J., Groshong, C., Harvey, A., Hickey, M.J., Hughes, N.E., Jones, S.A., Kim, E.J., Kuklish, S.L., Luz, J.G., Norman, B.H., Rathmell, R.E., Rizzo, J.R., Seng, T.W., Thibodeaux, S.J., Woods, T.A., York, J.S., Yu, X.P.(2016) J Med Chem 59: 194-205
- PubMed: 26653180
- DOI: https://doi.org/10.1021/acs.jmedchem.5b01249
- Primary Citation of Related Structures:
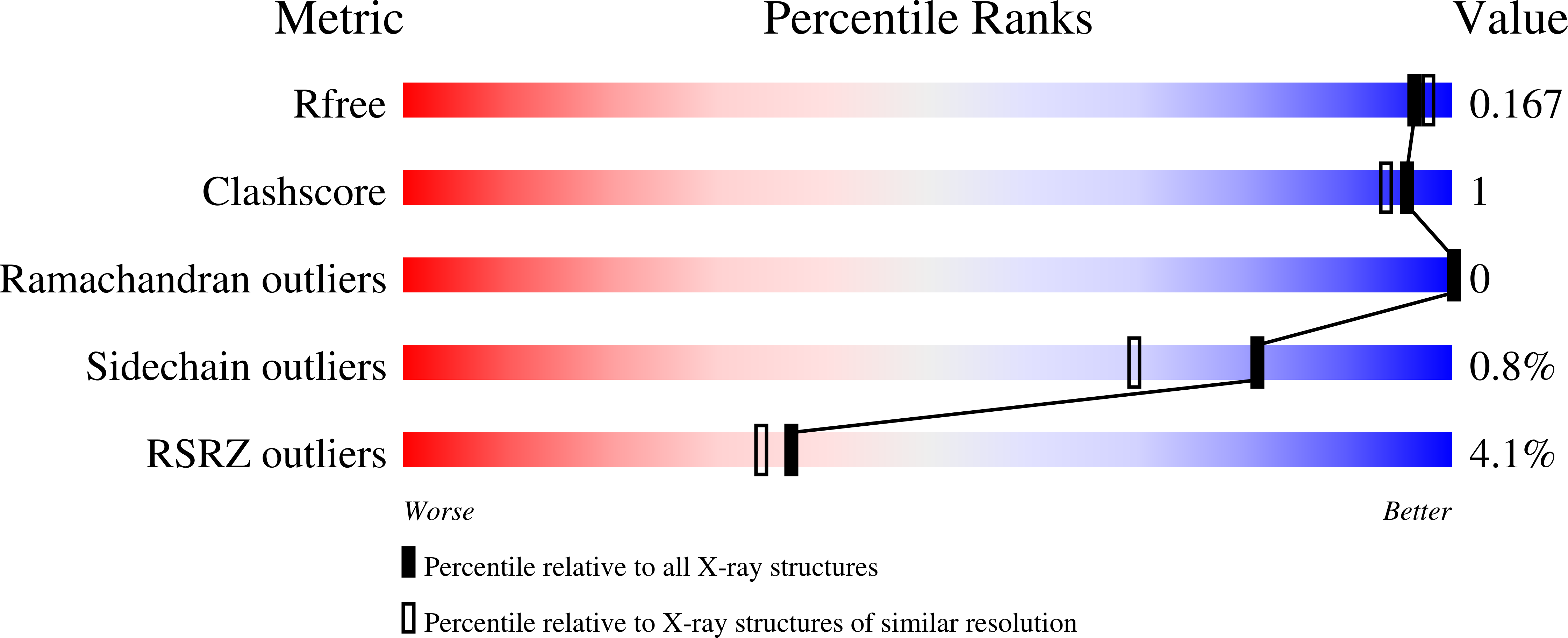
5BQG, 5BQH, 5BQI - PubMed Abstract:
As part of a program aimed at the discovery of antinociceptive therapy for inflammatory conditions, a screening hit was found to inhibit microsomal prostaglandin E synthase-1 (mPGES-1) with an IC50 of 17.4 μM. Structural information was used to improve enzyme potency by over 1000-fold. Addition of an appropriate substituent alleviated time-dependent cytochrome P450 3A4 (CYP3A4) inhibition. Further structure-activity relationship (SAR) studies led to 8, which had desirable potency (IC50 = 12 nM in an ex vivo human whole blood (HWB) assay) and absorption, distribution, metabolism, and excretion (ADME) properties. Studies on the formulation of 8 identified 8·H3PO4 as suitable for clinical development. Omission of a lipophilic portion of the compound led to 26, a readily orally bioavailable inhibitor with potency in HWB comparable to celecoxib. Furthermore, 26 was selective for mPGES-1 inhibition versus other mechanisms in the prostanoid pathway. These factors led to the selection of 26 as a second clinical candidate.
Organizational Affiliation:
Lilly Research Laboratories, A Division of Eli Lilly and Company , Indianapolis, Indiana 46285, United States.