The Complex Structure of mutant Phytase with IHS
Wu, T.H., Chen, C.C., Huang, C.H., Guo, R.T.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Periplasmic AppA protein | 410 | Escherichia coli | Mutation(s): 3 EC: 3.1.3.2 (PDB Primary Data), 3.1.3.26 (PDB Primary Data) | 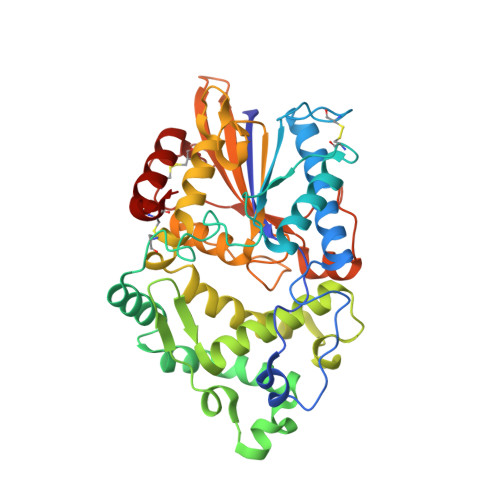 | |
UniProt | |||||
Find proteins for P07102 (Escherichia coli (strain K12)) Explore P07102 Go to UniProtKB: P07102 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P07102 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| IHS Query on IHS | F [auth A] | D-MYO-INOSITOL-HEXASULPHATE C6 H12 O24 S6 NBTMNFYXJYCQHQ-GPIVLXJGSA-N |  | ||
| NI Query on NI | B [auth A], C [auth A], D [auth A], E [auth A] | NICKEL (II) ION Ni VEQPNABPJHWNSG-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 63.796 | α = 90 |
| b = 47.5 | β = 100.51 |
| c = 65.632 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |