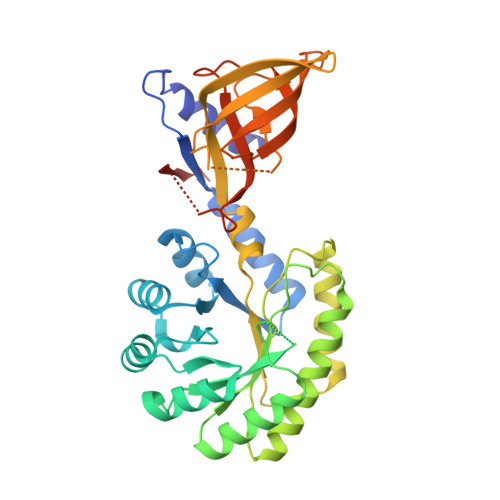
Structural Insight Into Dfmo Resistant Ornithine Decarboxylase from Entamoeba Histolytica: An Inkling to Adaptive Evolution.
Preeti, P., Tapas, S., Kumar, P., Tomar, S.(2013) PLoS One 8: 53397
- PubMed: 23326423
- DOI: https://doi.org/10.1371/journal.pone.0053397
- Primary Citation of Related Structures:
4AIB - PubMed Abstract:
Polyamine biosynthetic pathway is a validated therapeutic target for large number of infectious diseases including cancer, giardiasis and African sleeping sickness, etc. α-Difluoromethylornithine (DFMO), a potent drug used for the treatment of African sleeping sickness is an irreversible inhibitor of ornithine decarboxylase (ODC), the first rate limiting enzyme of polyamine biosynthesis. The enzyme ODC of E. histolytica (EhODC) has been reported to exhibit resistance towards DFMO. The basis for insensitivity towards DFMO was investigated by structural analysis of EhODC and conformational modifications at the active site. Here, we report cloning, purification and crystal structure determination of C-terminal truncated Entamoeba histolytica ornithine decarboxylase (EhODCΔ15). Structure was determined by molecular replacement method and refined to 2.8 Å resolution. The orthorhombic crystal exhibits P2(1)2(1)2(1) symmetry with unit cell parameters a = 76.66, b = 119.28, c = 179.28 Å. Functional as well as evolutionary relations of EhODC with other ODC homologs were predicted on the basis of sequence analysis, phylogeny and structure. We determined the tetrameric crystal structure of EhODCΔ15, which exists as a dimer in solution. Insensitivity towards DFMO is due to substitution of key substrate binding residues in active site pocket. Additionally, a few more substitutions similar to antizyme inhibitor (AZI), a non-functional homologue of ODCs, were identified in the active site. Here, we establish the fact that EhODC sequence has conserved PLP binding residues; in contrast few substrate binding residues are mutated similar to AZI. Further sequence analysis and structural studies revealed that EhODC may represent as an evolutionary bridge between active decarboxylase and inactive AZI.
Organizational Affiliation:
Department of Biotechnology, Indian Institute of Technology Roorkee, Roorkee, Uttarakhand, India.