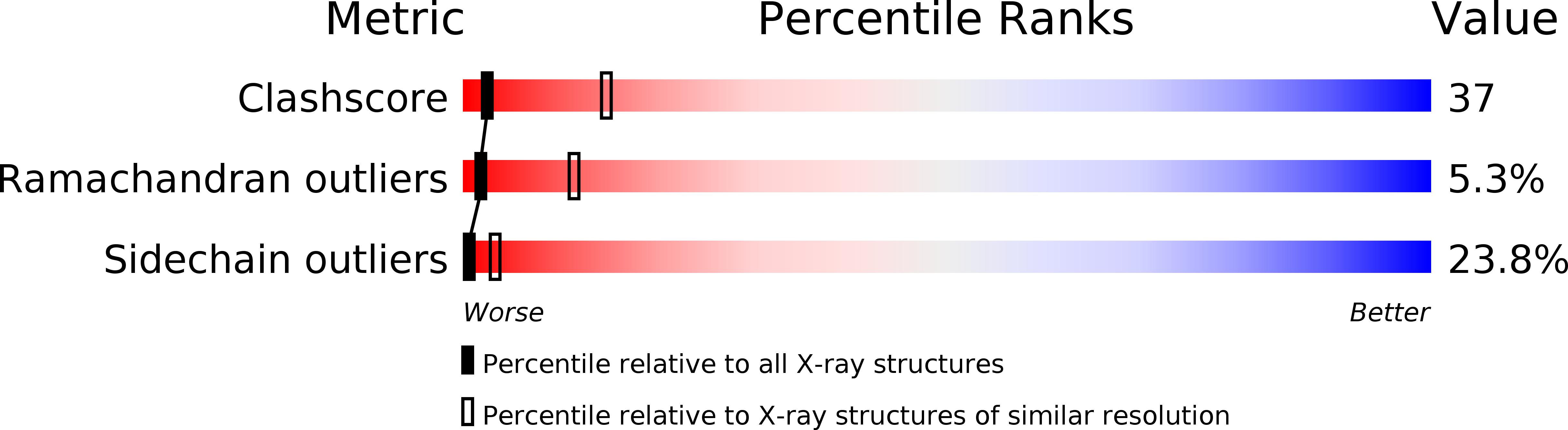The crystal structure of EcoRV endonuclease and of its complexes with cognate and non-cognate DNA fragments.
Winkler, F.K., Banner, D.W., Oefner, C., Tsernoglou, D., Brown, R.S., Heathman, S.P., Bryan, R.K., Martin, P.D., Petratos, K., Wilson, K.S.(1993) EMBO J 12: 1781-1795
- PubMed: 8491171
- DOI: https://doi.org/10.2210/pdb4rve/pdb
- Primary Citation of Related Structures:
1RVE, 2RVE, 4RVE - PubMed Abstract:
The crystal structure of EcoRV endonuclease has been determined at 2.5 A resolution and that of its complexes with the cognate DNA decamer GGGATATCCC (recognition sequence underlined) and the non-cognate DNA octamer CGAGCTCG at 3.0 A resolution. Two octamer duplexes of the non-cognate DNA, stacked end-to-end, are bound to the dimeric enzyme in B-DNA-like conformations. The protein--DNA interactions of this complex are prototypic for non-specific DNA binding. In contrast, only one cognate decamer duplex is bound and deviates considerably from canonical B-form DNA. Most notably, a kink of approximately 50 degrees is observed at the central TA step with a concomitant compression of the major groove. Base-specific hydrogen bonds between the enzyme and the recognition base pairs occur exclusively in the major groove. These interactions appear highly co-operative as they are all made through one short surface loop comprising residues 182-186. Numerous contacts with the sugar phosphate backbone extending beyond the recognition sequence are observed in both types of complex. However, the total surface area buried on complex formation is > 1800 A2 larger in the case of cognate DNA binding. Two acidic side chains, Asp74 and Asp90, are close to the reactive phosphodiester group in the cognate complex and most probably provide oxygen ligands for binding the essential cofactor Mg2+. An important role is also indicated for Lys92, which together with the two acidic functions appears to be conserved in the otherwise unrelated structure of EcoRI endonuclease. The structural results give new insight into the physical basis of the remarkable sequence specificity of this enzyme.
Organizational Affiliation:
Pharma Research-New Technologies, F. Hoffmann-La Roche Ltd, Basel, Switzerland.