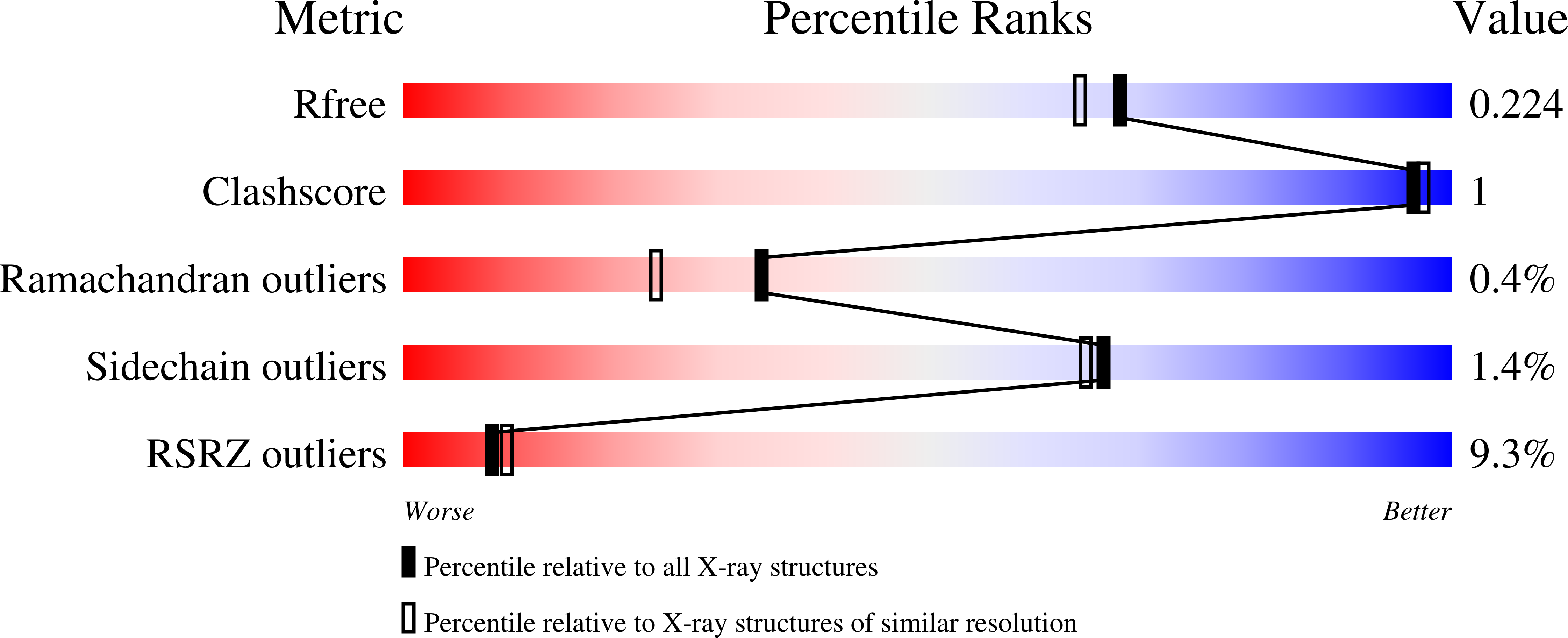
Rational design of inhibitors of the bacterial cell wall synthetic enzyme GlmU using virtual screening and lead-hopping.
Doig, P., Boriack-Sjodin, P.A., Dumas, J., Hu, J., Itoh, K., Johnson, K., Kazmirski, S., Kinoshita, T., Kuroda, S., Sato, T.O., Sugimoto, K., Tohyama, K., Aoi, H., Wakamatsu, K., Wang, H.(2014) Bioorg Med Chem 22: 6256-6269
- PubMed: 25262942
- DOI: https://doi.org/10.1016/j.bmc.2014.08.017
- Primary Citation of Related Structures:
4KNR, 4KNX, 4KPX, 4KPZ, 4KQL - PubMed Abstract:
An aminoquinazoline series targeting the essential bacterial enzyme GlmU (uridyltransferase) were previously reported (Biochem. J.2012, 446, 405). In this study, we further explored SAR through a combination of traditional medicinal chemistry and structure-based drug design, resulting in a novel scaffold (benzamide) with selectivity against protein kinases. Virtual screening identified fragments that could be fused into the core scaffold, exploiting additional binding interactions and thus improving potency. These efforts resulted in a hybrid compound with target potency increased by a 1000-fold, while maintaining selectivity against selected protein kinases and an improved level of solubility and protein binding. Despite these significant improvements no significant antibacterial activity was yet observed within this class.
Organizational Affiliation:
Discovery Sciences, AstraZeneca R&D Boston, 35 Gatehouse Drive, Waltham, MA 02451, United States. Electronic address: Peter.Doig@astrazeneca.com.