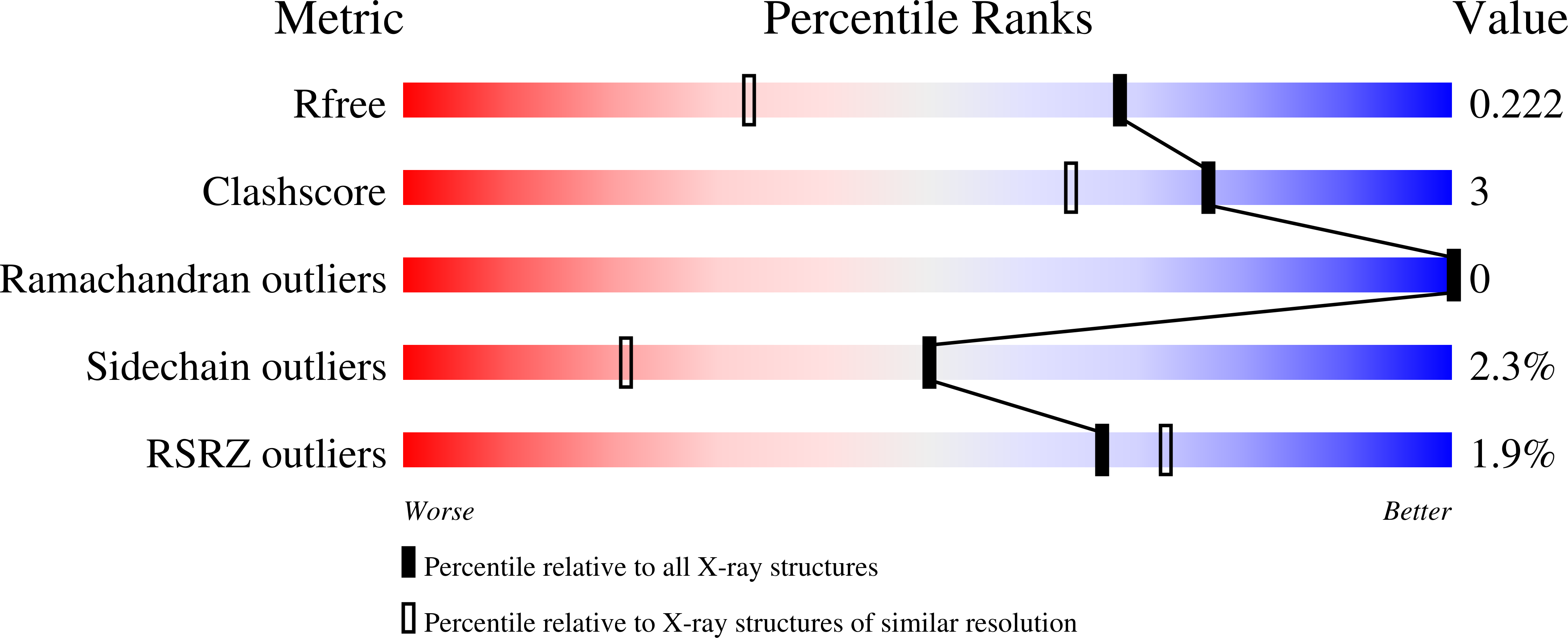
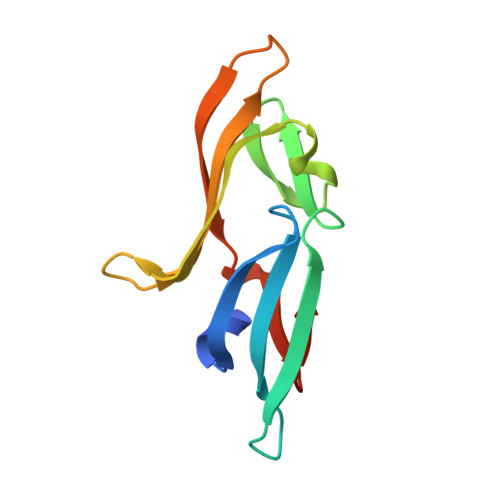
A designed chimeric cyanovirin-N homolog lectin: Structure and molecular basis of sucrose binding.
Koharudin, L.M., Furey, W., Gronenborn, A.M.(2009) Proteins 77: 904-915
- PubMed: 19639634
- DOI: https://doi.org/10.1002/prot.22514
- Primary Citation of Related Structures:
3HNU, 3HNX, 3HP8 - PubMed Abstract:
The NMR and X-ray structures of a designed chimeric cyanovirin-N homolog (CVNH) protein were determined. The individual halves of the structure are similar to their counterparts in the parent proteins, with domains A and B resembling the structures of TbCVNH and NcCVNH, respectively. No significant differences between the solution and crystal conformations were observed, although details in loop conformations and distinct crystal packing-induced features are present. Carbohydrate binding studies by NMR revealed affinity and specificity for Glc alpha(1-2)Frc and Man alpha(1-2)Man, and the parental half that is devoid of any sucrose affinity in NcCVNH was transformed into a genuine sucrose binding site in the context of the chimera. The atomic details of sugar recognition are seen in the crystal structure of the protein with two bound Glc alpha(1-2)Frc molecules. Both sugars exhibit different conformations around the glycosidic bond and engage in unique hydrogen bonding networks in the two sites. Although the protein is able to bind two Man alpha(1-2)Man molecules, a property associated with HIV-inactivation, no anti-HIV activity was observed for the hybrid protein. These results provide the structural basis for sugar recognition in the CVNH family and aid in deciphering the relationship between sugar binding and anti-HIV activity.
Organizational Affiliation:
Department of Structural Biology, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania 15260, USA.