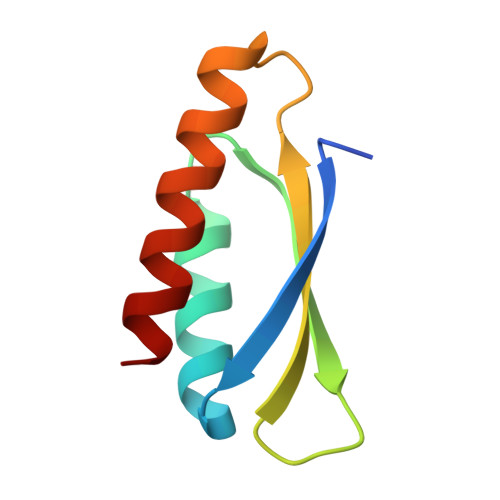
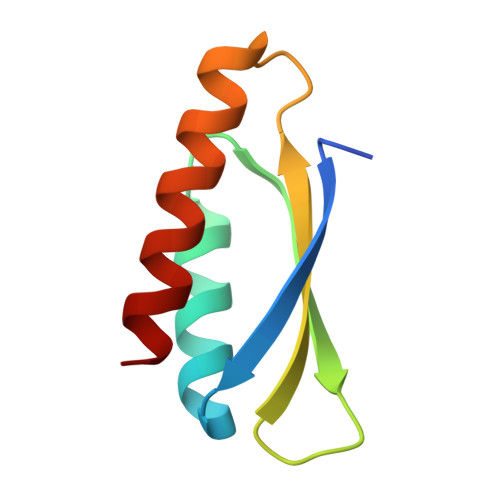
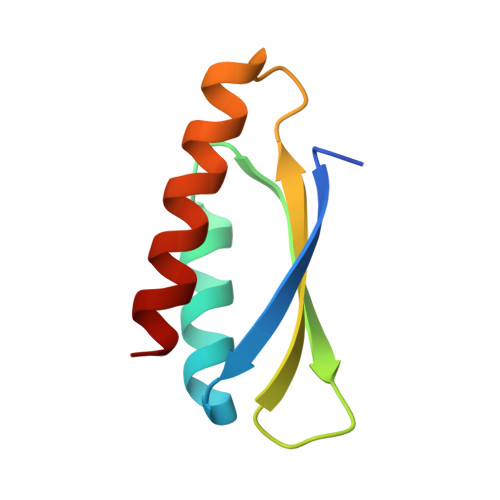
Trimming Down a Protein Structure to its Bare Foldons: Spatial Organization of the Cooperative Unit.
Haglund, E., Danielsson, J., Kadhirvel, S., Lindberg, M.O., Logan, D.T., Oliveberg, M.(2012) J Biological Chem 287: 2731
- PubMed: 22117065
- DOI: https://doi.org/10.1074/jbc.M111.312447
- Primary Citation of Related Structures:
3ZZP - PubMed Abstract:
Folding of the ribosomal protein S6 is a malleable process controlled by two competing, and partly overlapping, folding nuclei. Together, these nuclei extend over most of the S6 structure, except the edge strand β2, which is consistently missing in the folding transition states; despite being part of the S6 four-stranded sheet, β2 seems not to be part of the cooperative unit of the protein. The question is then whether β2 can be removed from the S6 structure without compromising folding cooperativity or native state integrity. To investigate this, we constructed a truncated variant of S6 lacking β2, reducing the size of the protein from 96 to 76 residues (S6(Δβ2)). The new S6 variant expresses well in Escherichia coli and has a well dispersed heteronuclear single quantum correlation spectrum and a perfectly wild-type-like crystal structure, but with a smaller three-stranded β-sheet. Moreover, S6(Δβ2) displays an archetypical v-shaped chevron plot with decreased slope of the unfolding limb, as expected from a protein with maintained folding cooperativity and reduced size. The results support the notion that foldons, as defined by the structural distribution of the folding nuclei, represent a property-based level of hierarchy in the build-up of larger protein structures and suggest that the role of β2 in S6 is mainly in intermolecular binding, consistent with the position of this strand in the ribosomal assembly.
Organizational Affiliation:
Department of Biochemistry and Biophysics, Arrhenius Laboratories of Natural Sciences, Stockholm University, S-106 91 Stockholm, Sweden.