Crystal structure of Alicyclobacillus acidocaldarius protein with beta-lactamase and rhodanese domains
Michalska, K., Chhor, G., Mandel, M.E., Bearden, J., Joachimiak, A., Midwest Center for Structural Genomics (MCSG)To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| BETA-LACTAMASE and RHODANESE DOMAIN PROTEIN | 474 | Alicyclobacillus acidocaldarius subsp. acidocaldarius DSM 446 | Mutation(s): 0 Gene Names: Aaci_0383 | 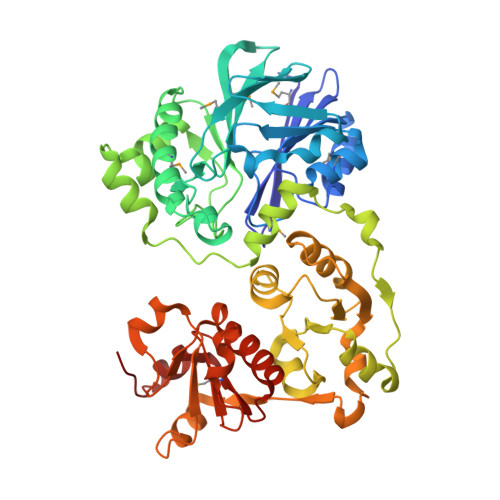 | |
UniProt | |||||
Find proteins for C8WS08 (Alicyclobacillus acidocaldarius subsp. acidocaldarius (strain ATCC 27009 / DSM 446 / BCRC 14685 / JCM 5260 / KCTC 1825 / NBRC 15652 / NCIMB 11725 / NRRL B-14509 / 104-IA)) Explore C8WS08 Go to UniProtKB: C8WS08 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | C8WS08 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ZN Query on ZN | C [auth A], D [auth B] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A, B | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 169.335 | α = 90 |
| b = 169.335 | β = 90 |
| c = 77.797 | γ = 90 |
| Software Name | Purpose |
|---|---|
| SBC-Collect | data collection |
| SHELX | model building |
| MLPHARE | phasing |
| DM | model building |
| ARP/wARP | model building |
| Coot | model building |
| REFMAC | refinement |
| HKL-3000 | data reduction |
| HKL-3000 | data scaling |
| SHELX | phasing |
| DM | phasing |