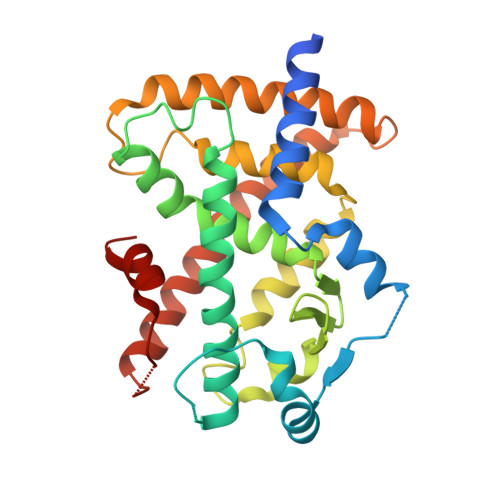
Discovery of GSK1997132B a novel centrally penetrant benzimidazole PPARgamma partial agonist.
Sime, M., Allan, A.C., Chapman, P., Fieldhouse, C., Giblin, G.M., Healy, M.P., Lambert, M.H., Leesnitzer, L.M., Lewis, A., Merrihew, R.V., Rutter, R.A., Sasse, R., Shearer, B.G., Wilson, T.M., Xu, R.X., Virley, D.J.(2011) Bioorg Med Chem Lett 21: 5568-5572
- PubMed: 21798739
- DOI: https://doi.org/10.1016/j.bmcl.2011.06.088
- Primary Citation of Related Structures:
3S9S - PubMed Abstract:
The peroxisome proliferator-activated receptor γ (PPARγ) is a ligand-activated nuclear receptor, thought to play a role in energy metabolism, glucose homeostasis and microglia-mediated neuroinflammation. A novel benzimidazole series of centrally penetrant PPARγ partial agonists has been identified. The optimization of PPARγ activity and in vivo pharmacokinetics leading to the identification of GSK1997132B a potent, metabolically stable and centrally penetrant PPARγ partial agonist, is described.
Organizational Affiliation:
Neurosciences Centre for Excellence in Drug Discovery, GlaxoSmithKline, New Frontiers Science Park, Harlow, Essex, CM19 5AW, United Kingdom. m.sime@beatson.gla.ac.uk