Insights into Substrate Gating in H. influenzae Rhomboid.
Brooks, C.L., Lazareno-Saez, C., Lamoureux, J.S., Mak, M.W., Lemieux, M.J.(2011) J Mol Biology 407: 687-697
- PubMed: 21295583
- DOI: https://doi.org/10.1016/j.jmb.2011.01.046
- Primary Citation of Related Structures:
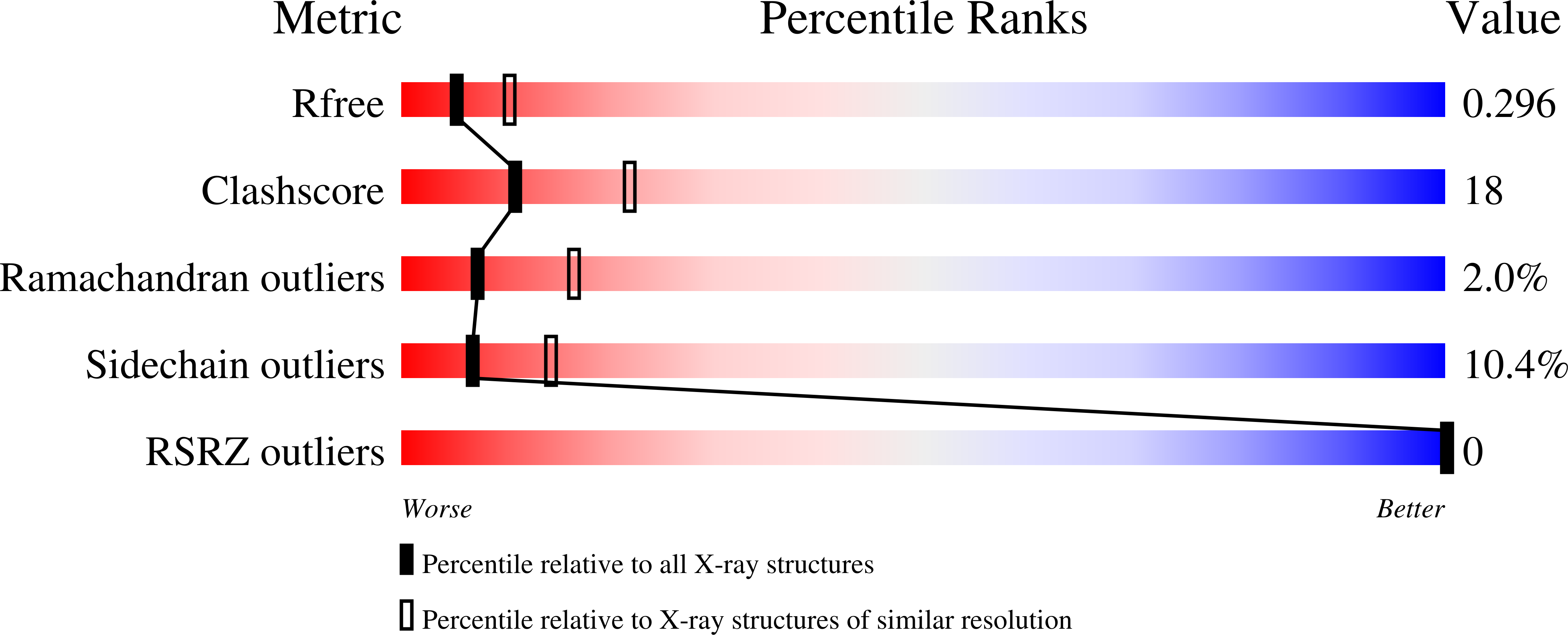
3ODJ - PubMed Abstract:
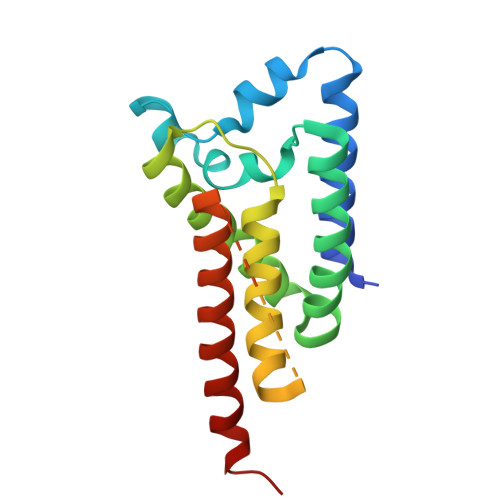
Rhomboids are a remarkable class of serine proteases that are embedded in lipid membranes. These membrane-bound enzymes play key roles in cellular signaling events, and disruptions in these events can result in numerous disease pathologies, including hereditary blindness, type 2 diabetes, Parkinson's disease, and epithelial cancers. Recent crystal structures of rhomboids from Escherichia coli have focused on how membrane-bound substrates gain access to a buried active site. In E. coli, it has been shown that movements of loop 5, with smaller movements in helix 5 and loop 4, act as substrate gate, facilitating inhibitor access to rhomboid catalytic residues. Herein we present a new structure of the Haemophilus influenzae rhomboid hiGlpG, which reveals disorder in loop 5, helix 5, and loop 4, indicating that, together, they represent mobile elements of the substrate gate. Substrate cleavage assays by hiGlpG with amino acid substitutions in these mobile regions demonstrate that the flexibilities of both loop 5 and helix 5 are important for access of the substrates to the catalytic residues. Mutagenesis indicates that less mobility by loop 4 is required for substrate cleavage. A reexamination of the reaction mechanism of rhomboid substrates, whereby cleavage of the scissile bond occurs on the si-face of the peptide bond, is discussed.
Organizational Affiliation:
Membrane Protein Disease Research Group, Department of Biochemistry, Faculty of Medicine and Dentistry,University of Alberta, Edmonton, Alberta, Canada T6G 2H7.