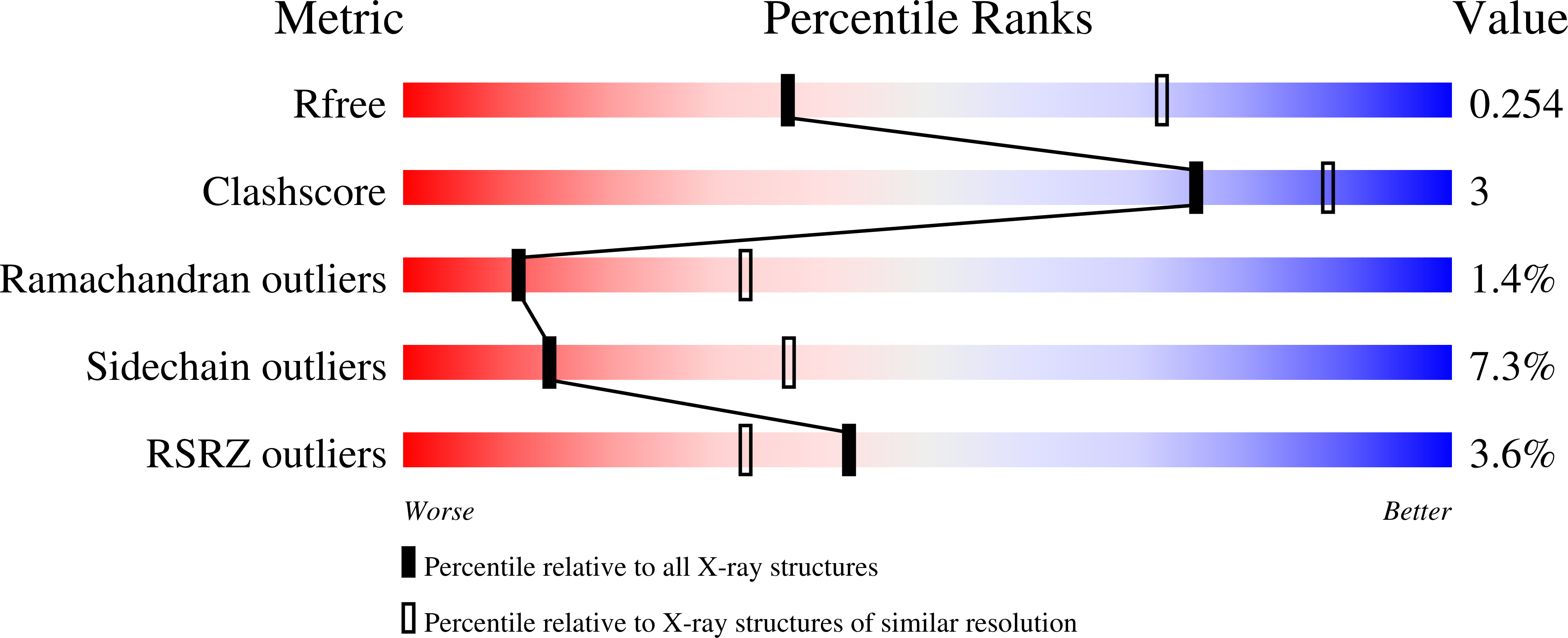
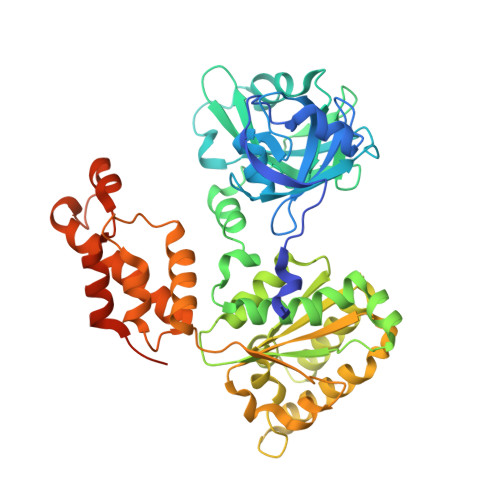
A novel ATP-dependent conformation in p97 N-D1 fragment revealed by crystal structures of disease-related mutants.
Tang, W.K., Li, D., Li, C.C., Esser, L., Dai, R., Guo, L., Xia, D.(2010) EMBO J 29: 2217-2229
- PubMed: 20512113
- DOI: https://doi.org/10.1038/emboj.2010.104
- Primary Citation of Related Structures:
3HU1, 3HU2, 3HU3 - PubMed Abstract:
Mutations in p97, a major cytosolic AAA (ATPases associated with a variety of cellular activities) chaperone, cause inclusion body myopathy associated with Paget's disease of the bone and frontotemporal dementia (IBMPFD). IBMPFD mutants have single amino-acid substitutions at the interface between the N-terminal domain (N-domain) and the adjacent AAA domain (D1), resulting in a reduced affinity for ADP. The structures of p97 N-D1 fragments bearing IBMPFD mutations adopt an atypical N-domain conformation in the presence of Mg(2+).ATPgammaS, which is reversible by ADP, showing for the first time the nucleotide-dependent conformational change of the N-domain. The transition from the ADP- to the ATPgammaS-bound state is accompanied by a loop-to-helix conversion in the N-D1 linker and by an apparent re-ordering in the N-terminal region of p97. X-ray scattering experiments suggest that wild-type p97 subunits undergo a similar nucleotide-dependent N-domain conformational change. We propose that IBMPFD mutations alter the timing of the transition between nucleotide states by destabilizing the ADP-bound form and consequently interfere with the interactions between the N-domains and their substrates.
Organizational Affiliation:
Laboratory of Cell Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.