Mouse Piwi interactome identifies binding mechanism of Tdrkh Tudor domain to arginine methylated Miwi
Chen, C., Jin, J., James, D.A., Adams-Cioaba, M.A., Park, J.G., Guo, Y., Tenaglia, E., Xu, C., Gish, G., Min, J., Pawson, T.(2009) Proc Natl Acad Sci U S A 106: 20336-20341
- PubMed: 19918066
- DOI: https://doi.org/10.1073/pnas.0911640106
- Primary Citation of Related Structures:
3FDR - PubMed Abstract:
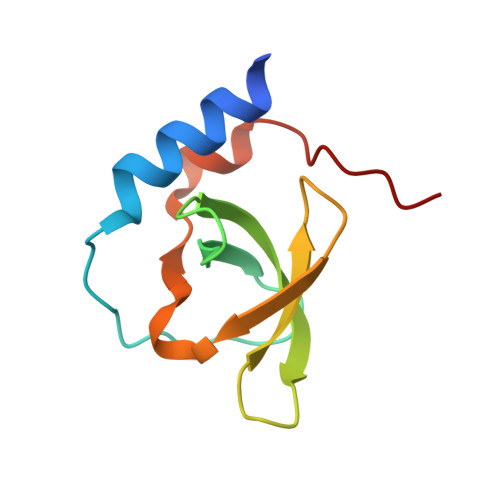
Tudor domains are protein modules that mediate protein-protein interactions, potentially by binding to methylated ligands. A group of germline specific single and multiTudor domain containing proteins (TDRDs) represented by drosophila Tudor and its mammalian orthologs Tdrd1, Tdrd4/RNF17, and Tdrd6 play evolutionarily conserved roles in germinal granule/nuage formation and germ cell specification and differentiation. However, their physiological ligands, and the biochemical and structural basis for ligand recognition, are largely unclear. Here, by immunoprecipitation of endogenous murine Piwi proteins (Miwi and Mili) and proteomic analysis of complexes related to the piRNA pathway, we show that the TDRD group of Tudor proteins are physiological binding partners of Piwi family proteins. In addition, mass spectrometry indicates that arginine residues in RG repeats at the N-termini of Miwi and Mili are methylated in vivo. Notably, we found that Tdrkh/Tdrd2, a novel single Tudor domain containing protein identified in the Miwi complex, is expressed in the cytoplasm of male germ cells and directly associates with Miwi. Mutagenesis studies mapped the Miwi-Tdrkh interaction to the very N-terminal RG/RA repeats of Miwi and showed that the Tdrkh Tudor domain is critical for binding. Furthermore, we have solved the crystal structure of the Tdrkh Tudor domain, which revealed an aromatic binding pocket and negatively charged binding surface appropriate for accommodating methylated arginine. Our findings identify a methylation-directed protein interaction mechanism in germ cells mediated by germline Tudor domains and methylated Piwi family proteins, and suggest a complex mode of regulating the organization and function of Piwi proteins in piRNA silencing pathways.
Organizational Affiliation:
Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, ON, Canada M5G 1X5.