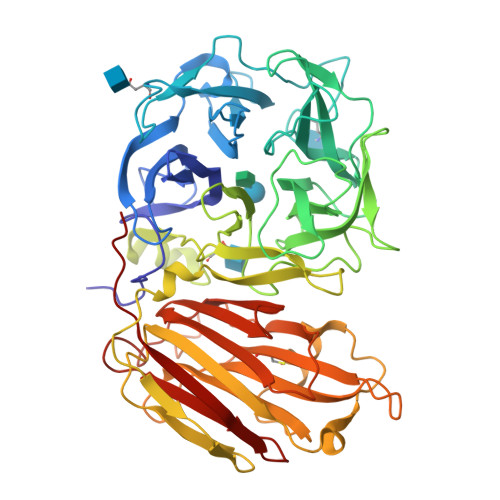
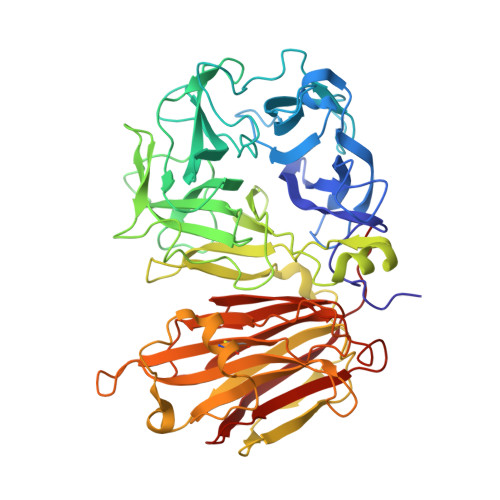
An alternate sucrose binding mode in the E203Q Arabidopsis invertase mutant: An X-ray crystallography and docking study.
Matrai, J., Lammens, W., Jonckheer, A., Le Roy, K., Rabijns, A., Van den Ende, W., De Maeyer, M.(2007) Proteins 71: 552-564
- PubMed: 17963237
- DOI: https://doi.org/10.1002/prot.21700
- Primary Citation of Related Structures:
2OXB - PubMed Abstract:
In the present study, we report on the X-ray crystallographic structure of a GH32 invertase mutant, (i.e., the Arabidopsis thaliana cell-wall invertase 1-E203Q, AtcwINV1-mutant) in complex with sucrose. This structure was solved to reveal the features of sugar binding in the catalytic pocket. However, as demonstrated by the X-ray structure the sugar binding and the catalytic pocket arrangement is significantly altered as compared with what was expected based on previous X-ray structures on GH-J clan enzymes. We performed a series of docking and molecular dynamics simulations on various derivatives of AtcwINV1 to reveal the reasons behind this modified sugar binding. Our results demonstrate that the E203Q mutation introduced into the catalytic pocket triggers conformational changes that alter the wild type substrate binding. In addition, this study also reveals the putative productive sucrose binding modus in the wild type enzyme.
Organizational Affiliation:
Laboratory of Biomolecular Modelling and BioMacS, Department of Chemistry, Katholieke Universiteit Leuven, Belgium.