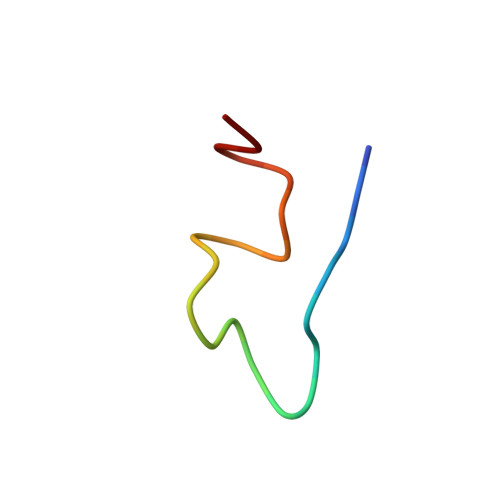
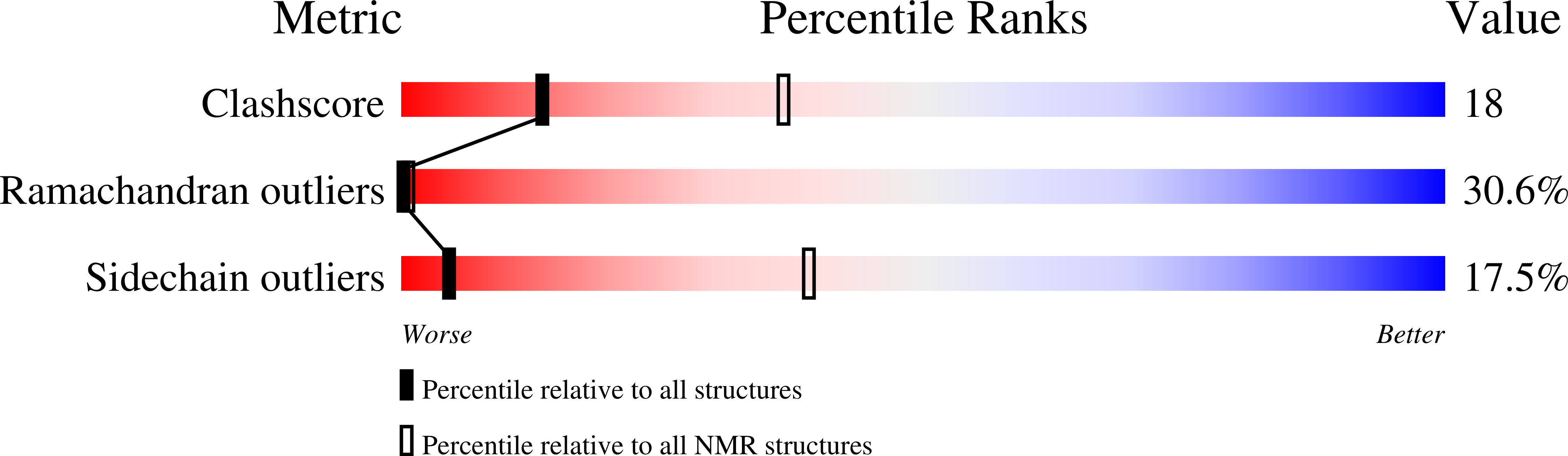NMR structure of the viral peptide linked to the genome (VPg) of poliovirus.
Schein, C.H., Oezguen, N., Volk, D.E., Garimella, R., Paul, A., Braun, W.(2006) Peptides 27: 1676-1684
- PubMed: 16540201
- DOI: https://doi.org/10.1016/j.peptides.2006.01.018
- Primary Citation of Related Structures:
2BBL, 2BBP - PubMed Abstract:
VPgs are essential for replication of picornaviruses, which cause diseases such as poliomyelitis, foot and mouth disease, and the common cold. VPg in infected cells is covalently linked to the 5' end of the viral RNA, or, in a uridylylated form, free in the cytoplasm. We show here the first solution structure for a picornaviral VPg, that of the 22-residue peptide from poliovirus serotype 1. VPg in buffer is inherently flexible, but a single conformer was obtained by adding trimethylamine N-oxide (TMAO). TMAO had only minor effects on the TOCSY spectrum. However, it increased the amount of structured peptide, as indicated by more peaks in the NOESY spectrum and an up to 300% increase in the ratio of normalized NOE cross peak intensities to that in buffer. The data for VPg in TMAO yielded a well defined structure bundle with 0.6 A RMSD (versus 6.6 A in buffer alone), with 10-30 unambiguous constraints per residue. The structure consists of a large loop region from residues 1 to 14, from which the reactive tyrosinate projects outward, and a C-terminal helix from residues 18 to 21 that aligns the sidechains of conserved residues on one face. The structure has a stable docking position at an area on the poliovirus polymerase crystal structure identified as a VPg binding site by mutagenesis studies. Further, UTP and ATP dock in a base-specific manner to the reactive face of VPg, held in place by residues conserved in all picornavirus VPgs.
Organizational Affiliation:
Sealy Center for Structural Biology and Molecular Biophysics, Department of Human Biochemistry and Molecular Biology, University of Texas Medical Branch, Galveston, TX 77555-0857, USA. chschein@utmb.edu