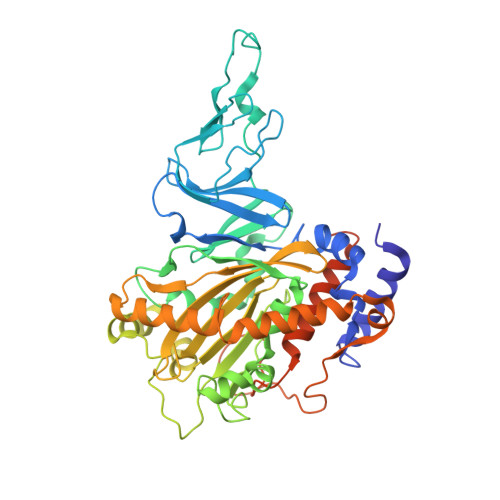
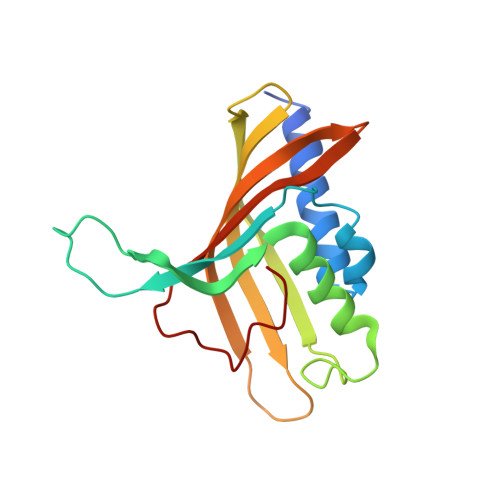
Structure and Increased Thermostability of Rhodococcus sp. Naphthalene 1,2-Dioxygenase.
Gakhar, L., Malik, Z.A., Allen, C.C., Lipscomb, D.A., Larkin, M.J., Ramaswamy, S.(2005) J Bacteriol 187: 7222-7231
- PubMed: 16237006
- DOI: https://doi.org/10.1128/JB.187.21.7222-7231.2005
- Primary Citation of Related Structures:
2B1X, 2B24 - PubMed Abstract:
Rieske nonheme iron oxygenases form a large class of aromatic ring-hydroxylating dioxygenases found in microorganisms. These enzymes enable microorganisms to tolerate and even exclusively utilize aromatic compounds for growth, making them good candidates for use in synthesis of chiral intermediates and bioremediation. Studies of the chemical stability and thermostability of these enzymes thus become important. We report here the structure of free and substrate (indole)-bound forms of naphthalene dioxygenase from Rhodococcus sp. strain NCIMB12038. The structure of the Rhodococcus enzyme reveals that, despite a approximately 30% sequence identity between these naphthalene dioxygenases, their overall structures superpose very well with a root mean square deviation of less than 1.6 A. The differences in the active site of the two enzymes are pronounced near the entrance; however, indole binds to the Rhodococcus enzyme in the same orientation as in the Pseudomonas enzyme. Circular dichroism spectroscopy experiments show that the Rhodococcus enzyme has higher thermostability than the naphthalene dioxygenase from Pseudomonas species. The Pseudomonas enzyme has an apparent melting temperature of 55 degrees C while the Rhodococcus enzyme does not completely unfold even at 95 degrees C. Both enzymes, however, show similar unfolding behavior in urea, and the Rhodococcus enzyme is only slightly more tolerant to unfolding by guanidine hydrochloride. Structure analysis suggests that the higher thermostability of the Rhodococcus enzyme may be attributed to a larger buried surface area and extra salt bridge networks between the alpha and beta subunits in the Rhodococcus enzyme.
Organizational Affiliation:
Department of Biochemistry, University of Iowa, Iowa City, IA 52242, USA.