Structural Characterization of the C3 Domain of Cardiac Myosin Binding Protein C and Its Hypertrophic Cardiomyopathy-Related R502W Mutant.
Zhang, X.L., De, S., McIntosh, L.P., Paetzel, M.(2014) Biochemistry 53: 5332-5342
- PubMed: 25058872
- DOI: https://doi.org/10.1021/bi500784g
- Primary Citation of Related Structures:
2MQ0, 2MQ3 - PubMed Abstract:
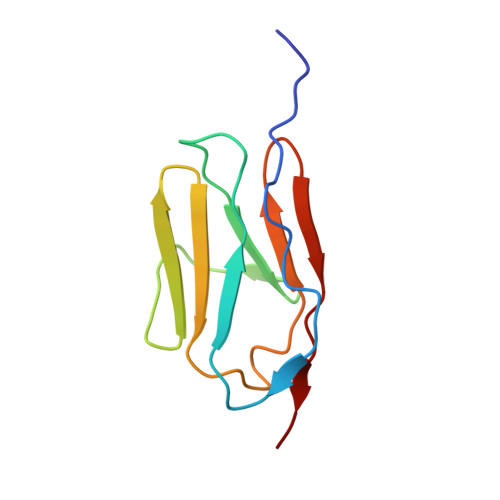
Human cardiac myosin binding protein C (cMyBP-C), a thick filament protein found within the sarcomere of cardiac muscle, regulates muscle contraction and is essential for proper muscle function. Hypertrophic cardiomyopathy (HCM), a genetic disease affecting 1 in 500 people, is the major cause of death in young athletes. It is caused by genetic mutations within sarcomeric proteins. Forty-two percent of the HCM-related mutations are found in cMyBP-C. Here we present the nuclear magnetic resonance-derived structural ensembles of the wild-type cMyBP-C C3 domain and its HCM-related R502W mutant. The C3 domain adopts an immunoglobulin-like fold, and mutation of the exposed Arg502 to a tryptophan does not perturb its structure, dynamics, or stability. However, the R502W mutation does alter the predicted electrostatic properties of the C3 domain. We hypothesize that this mutation, and other HCM-linked mutations found within the same domain, may directly disrupt the interaction of cMyBP-C with other sarcomeric proteins.
Organizational Affiliation:
Department of Molecular Biology and Biochemistry, Simon Fraser University , South Science Building, 8888 University Drive, Burnaby, British Columbia, Canada V5A 1S6.