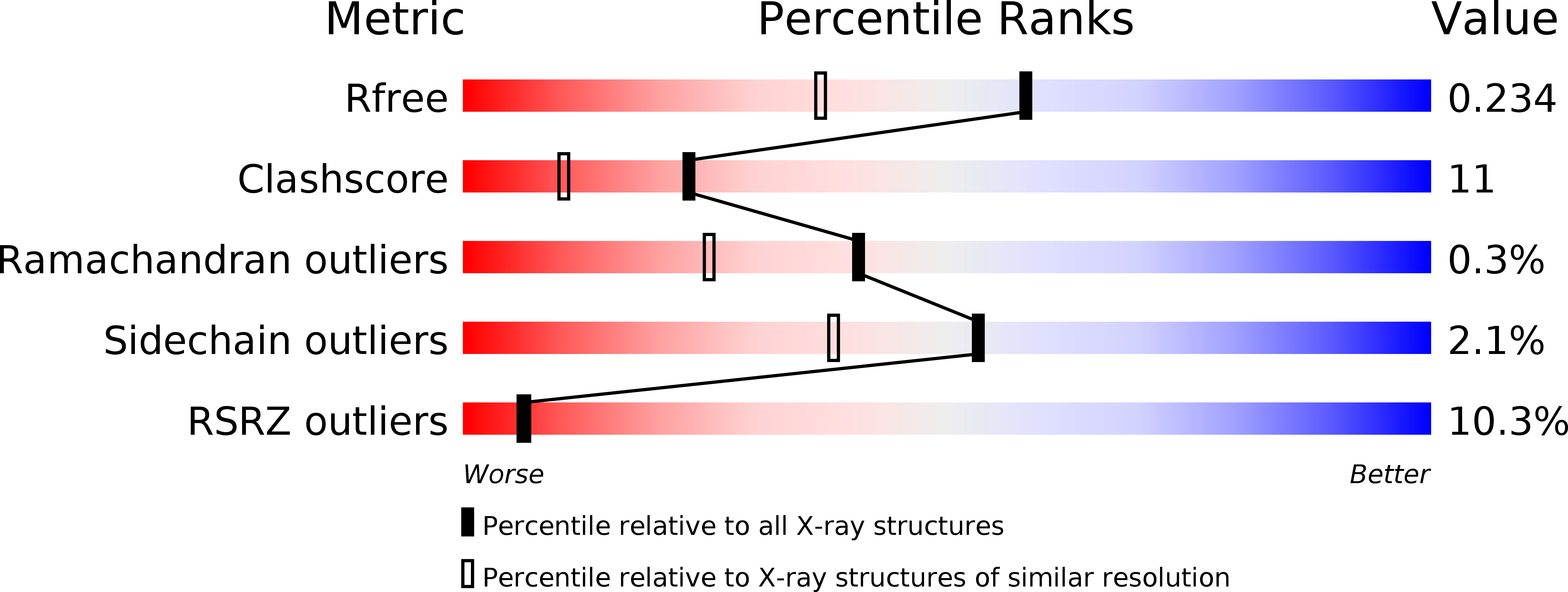
The structural basis of the catalytic mechanism and regulation of glucose-1-phosphate thymidylyltransferase (RmlA).
Blankenfeldt, W., Asuncion, M., Lam, J.S., Naismith, J.H.(2000) EMBO J 19: 6652-6663
- PubMed: 11118200
- DOI: https://doi.org/10.1093/emboj/19.24.6652
- Primary Citation of Related Structures:
1FXO, 1FZW, 1G0R, 1G1L, 1G23, 1G2V, 1G3L - PubMed Abstract:
The synthesis of deoxy-thymidine di-phosphate (dTDP)-L-rhamnose, an important component of the cell wall of many microorganisms, is a target for therapeutic intervention. The first enzyme in the dTDP-L-rhamnose biosynthetic pathway is glucose-1-phosphate thymidylyltransferase (RmlA). RmlA is inhibited by dTDP-L-rhamnose thereby regulating L-rhamnose production in bacteria. The structure of Pseudomonas aeruginosa RmlA has been solved to 1.66 A resolution. RmlA is a homotetramer, with the monomer consisting of three functional subdomains. The sugar binding and dimerization subdomains are unique to RmlA-like enzymes. The sequence of the core subdomain is found not only in sugar nucleotidyltransferases but also in other nucleotidyltransferases. The structures of five distinct enzyme substrate- product complexes reveal the enzyme mechanism that involves precise positioning of the nucleophile and activation of the electrophile. All the key residues are within the core subdomain, suggesting that the basic mechanism is found in many nucleotidyltransferases. The dTDP-L-rhamnose complex identifies how the protein is controlled by its natural inhibitor. This work provides a platform for the design of novel drugs against pathogenic bacteria.
Organizational Affiliation:
The Centre for Biomolecular Sciences, The University, St Andrews, KY16 9ST, UK.