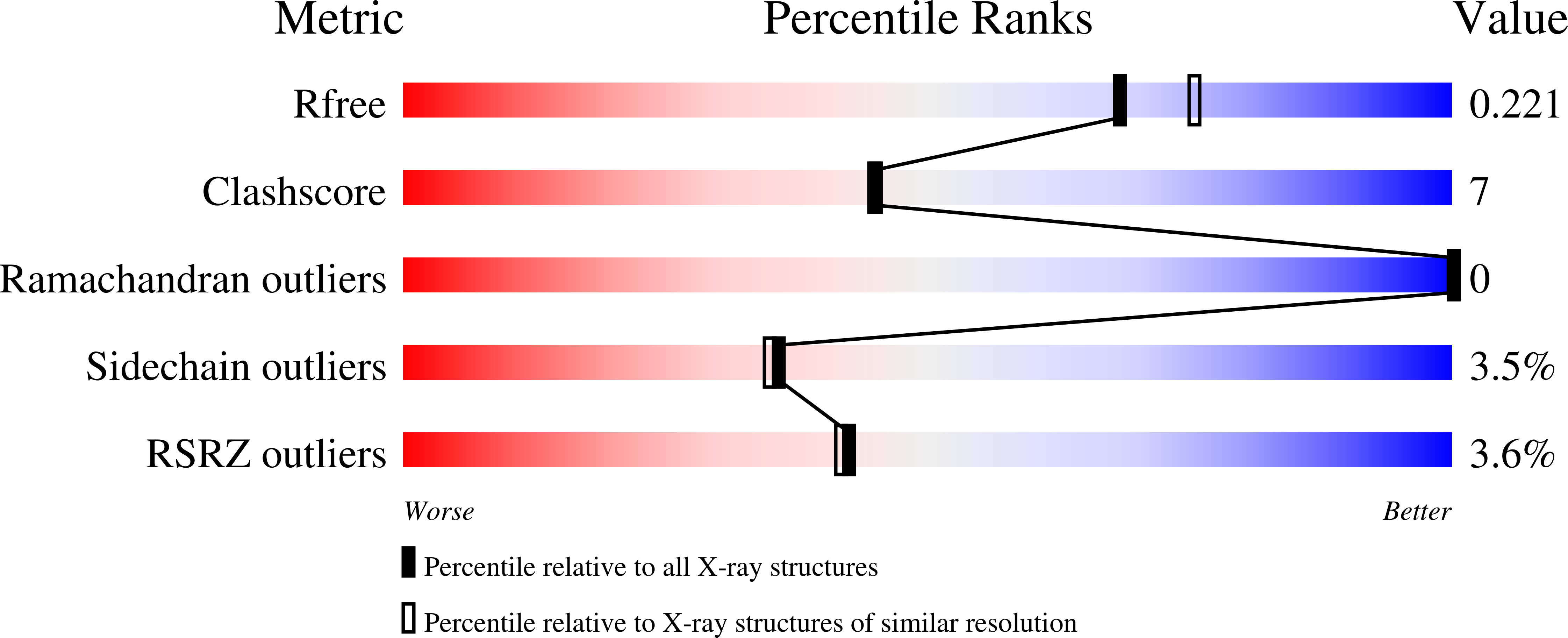
Structural basis for substrate specificity of the human mitochondrial deoxyribonucleotidase
Wallden, K., Ruzzenente, B., Rinaldo-Matthis, A., Bianchi, V., Nordlund, P.(2005) Structure 13: 1081-1088
- PubMed: 16004879
- DOI: https://doi.org/10.1016/j.str.2005.04.023
- Primary Citation of Related Structures:
1Z4I, 1Z4J, 1Z4K, 1Z4L, 1Z4M, 1Z4P, 1Z4Q - PubMed Abstract:
The human mitochondrial deoxyribonucleotidase catalyzes the dephosphorylation of thymidine and deoxyuridine monophosphates and participates in the regulation of the dTTP pool in mitochondria. We present seven structures of the inactive D41N variant of this enzyme in complex with thymidine 3'-monophosphate, thymidine 5'-monophosphate, deoxyuridine 5'-monophosphate, uridine 5'-monophosphate, deoxyguanosine 5'-monophosphate, uridine 2'-monophosphate, and the 5'-monophosphate of the nucleoside analog 3'-deoxy 2'3'-didehydrothymidine, and we draw conclusions about the substrate specificity based on comparisons with enzyme activities. We show that the enzyme's specificity for the deoxyribo form of nucleoside 5'-monophosphates is due to Ile-133, Phe-49, and Phe-102, which surround the 2' position of the sugar and cause an energetically unfavorable environment for the 2'-hydroxyl group of ribonucleoside 5'-monophosphates. The close binding of the 3'-hydroxyl group of nucleoside 5'-monophosphates to the enzyme indicates that nucleoside analog drugs that are substituted with a bulky group at this position will not be good substrates for this enzyme.
Organizational Affiliation:
Department of Biochemistry and Biophysics, Stockholm University, SE-106 91 Stockholm, Sweden.