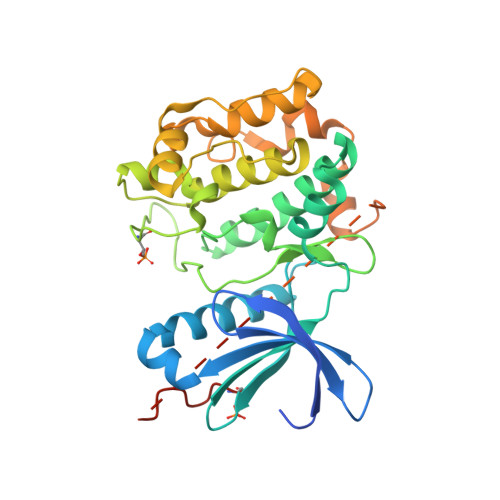
Catalytic domain crystal structure of protein kinase C-theta (PKCtheta)
Xu, Z.B., Chaudhary, D., Olland, S., Wolfrom, S., Czerwinski, R., Malakian, K., Lin, L., Stahl, M.L., Joseph-McCarthy, D., Benander, C., Fitz, L., Greco, R., Somers, W.S., Mosyak, L.(2004) J Biol Chem 279: 50401-50409
- PubMed: 15364937
- DOI: https://doi.org/10.1074/jbc.M409216200
- Primary Citation of Related Structures:
1XJD - PubMed Abstract:
A member of the novel protein kinase C (PKC) subfamily, PKC, is an essential component of the T cell synapse and is required for optimal T cell activation and interleukin-2 production. Selective involvement of PKC in TCR signaling makes this enzyme an attractive therapeutic target in T cell-mediated disease processes. In this report we describe the crystal structure of the catalytic domain of PKC at 2.0-A resolution. Human recombinant PKC kinase domain was expressed in bacteria as catalytically active phosphorylated enzyme and co-crystallized with its subnanomolar, ATP site inhibitor staurosporine. The structure follows the classic bilobal kinase fold and shows the enzyme in its active conformation and phosphorylated state. Inhibitory interactions between conserved features of staurosporine and the ATP-binding cleft are accompanied by closing of the glycine-rich loop, which also maintains an inhibitory arrangement by blocking the phosphate recognition subsite. The two major phosphorylation sites, Thr-538 in the activation loop and Ser-695 in the hydrophobic motif, are both occupied in the structure, playing key roles in stabilizing active conformation of the enzyme and indicative of PKC autocatalytic phosphorylation and activation during bacterial expression. The PKC-staurosporine complex represents the first kinase domain crystal structure of any PKC isotypes to be determined and as such should provide valuable insight into PKC specificity and into rational drug design strategies for PKC selective leads.
Organizational Affiliation:
Department of Chemical and Screening Sciences, Inflammation Department, Wyeth Research, Cambridge, Massachusetts 02140, USA.