Activation of the LicT Transcriptional Antiterminator Involves a Domain Swing/Lock Mechanism Provoking Massive Structural Changes
Graille, M., Zhou, C.-Z., Receveur-Brechot, V., Collinet, B., Declerck, N., van Tilbeurgh, H.(2005) J Biol Chem 280: 14780-14789
- PubMed: 15699035
- DOI: https://doi.org/10.1074/jbc.M414642200
- Primary Citation of Related Structures:
1TLV - PubMed Abstract:
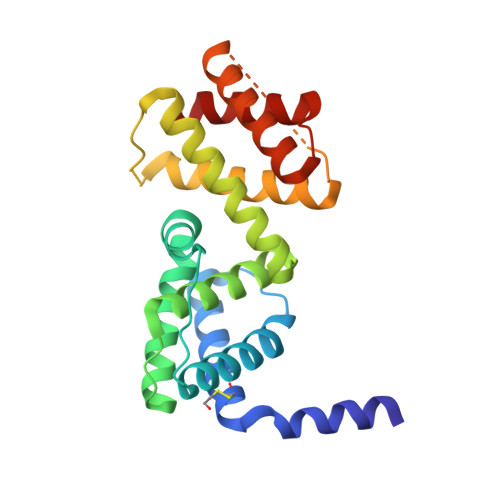
The transcriptional antiterminator protein LicT regulates the expression of Bacillus subtilis operons involved in beta-glucoside metabolism. It consists of an N-terminal RNA-binding domain (co-antiterminator (CAT)) and two phosphorylatable phosphotransferase system regulation domains (PRD1 and PRD2). In the activated state, each PRD forms a dimeric unit with the phosphorylation sites totally buried at the dimer interface. Here we present the 1.95 A resolution structure of the inactive LicT PRDs as well as the molecular solution structure of the full-length protein deduced from small angle x-ray scattering. Comparison of native (inactive) and mutant (constitutively active) PRD crystal structures shows massive tertiary and quaternary rearrangements of the entire regulatory domain. In the inactive state, a wide swing movement of PRD2 results in dimer opening and brings the phosphorylation sites to the protein surface. This movement is accompanied by additional structural rearrangements of both the PRD1-PRD1 ' interface and the CAT-PRD1 linker. Small angle x-ray scattering experiments indicate that the amplitude of the PRD2 swing might even be wider in solution than in the crystals. Our results suggest that PRD2 is highly mobile in the native protein, whereas it is locked upon activation by phosphorylation.
Organizational Affiliation:
Laboratoire d'Enzymologie et Biochimie Structurales, CNRS-UPR9063, Gif sur Yvette, France.