Comparison of beta-lactamases of classes A and D: 1.5A crystallographic structure of the class D OXA-1 oxacillinase
Sun, T., Nukaga, M., Mayama, K., Braswell, E.H., Knox, J.R.(2003) Protein Sci 12: 82-91
- PubMed: 12493831
- DOI: https://doi.org/10.1110/ps.0224303
- Primary Citation of Related Structures:
1M6K - PubMed Abstract:
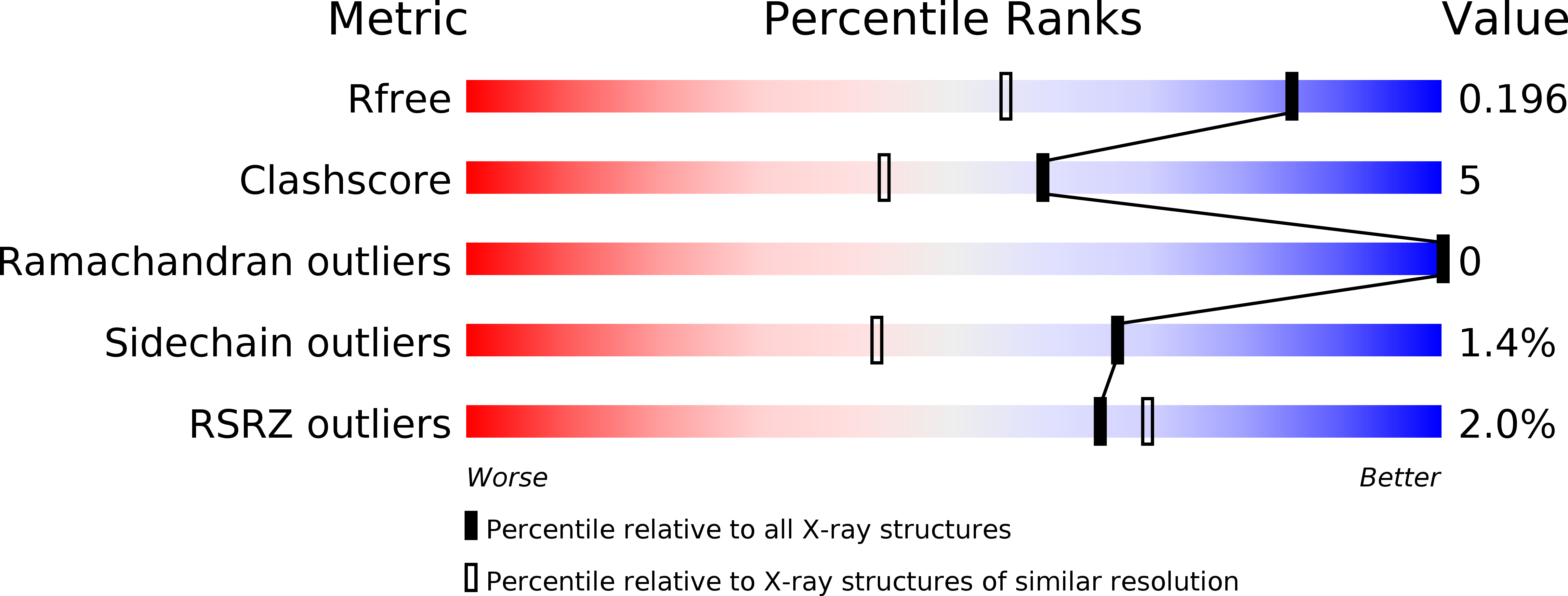
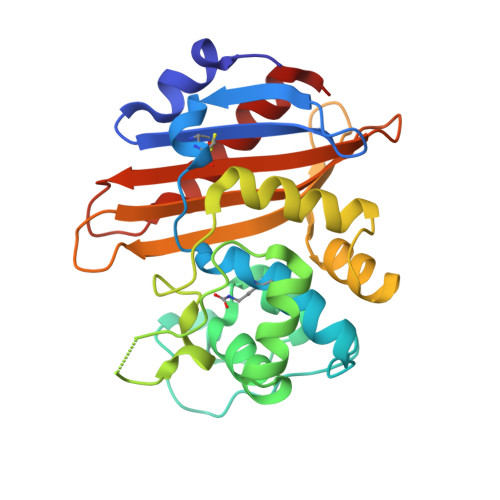
The crystallographic structure of the Escherichia coli OXA-1 beta-lactamase has been established at 1.5-A resolution and refined to R = 0.18. The 28.2-kD oxacillinase is a class D serine beta-lactamase that is especially active against the penicillin-type beta-lactams oxacillin and cloxacillin. In contrast to the structures of OXA-2, OXA-10, and OXA-13 belonging to other subclasses, the OXA-1 molecule is monomeric rather than dimeric and represents the subclass characterized by an enlarged Omega loop near the beta-lactam binding site. The 6-residue hydrophilic insertion in this loop cannot interact directly with substrates and, instead, projects into solvent. In this structure at pH 7.5, carboxylation of the conserved Lys 70 in the catalytic site is observed. One oxygen atom of the carboxylate group is hydrogen bonded to Ser 120 and Trp 160. The other oxygen atom is more exposed and hydrogen bonded to the Ogamma of the reactive Ser 67. In the overlay of the class D and class A binding sites, the carboxylate group is displaced ca. 2.6 A from the carboxylate group of Glu 166 of class A enzymes. However, each group is equidistant from the site of the water molecule expected to function in hydrolysis, and which could be activated by the carboxylate group of Lys 70. In this ligand-free OXA-1 structure, no water molecule is seen in this site, so the water molecule must enter after formation of the acyl-Ser 67 intermediate.
Organizational Affiliation:
Department of Molecular and Cell Biology, The University of Connecticut, Storrs, CT 06269, USA.