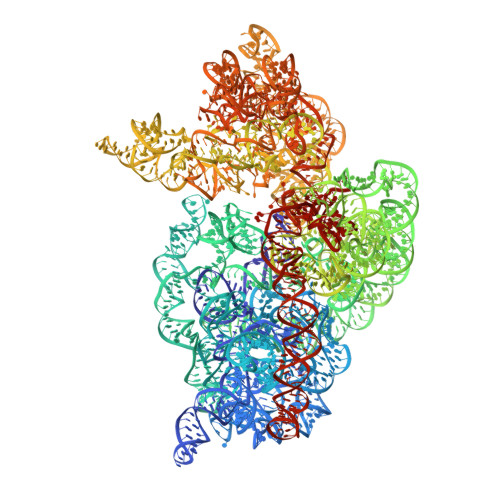
Recognition of cognate transfer RNA by the 30S ribosomal subunit.
Ogle, J.M., Brodersen, D.E., Clemons Jr., W.M., Tarry, M.J., Carter, A.P., Ramakrishnan, V.(2001) Science 292: 897-902
- PubMed: 11340196
- DOI: https://doi.org/10.1126/science.1060612
- Primary Citation of Related Structures:
1IBK, 1IBL, 1IBM - PubMed Abstract:
Crystal structures of the 30S ribosomal subunit in complex with messenger RNA and cognate transfer RNA in the A site, both in the presence and absence of the antibiotic paromomycin, have been solved at between 3.1 and 3.3 angstroms resolution. Cognate transfer RNA (tRNA) binding induces global domain movements of the 30S subunit and changes in the conformation of the universally conserved and essential bases A1492, A1493, and G530 of 16S RNA. These bases interact intimately with the minor groove of the first two base pairs between the codon and anticodon, thus sensing Watson-Crick base-pairing geometry and discriminating against near-cognate tRNA. The third, or "wobble," position of the codon is free to accommodate certain noncanonical base pairs. By partially inducing these structural changes, paromomycin facilitates binding of near-cognate tRNAs.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Hills Road, Cambridge CB2 2QH, UK.