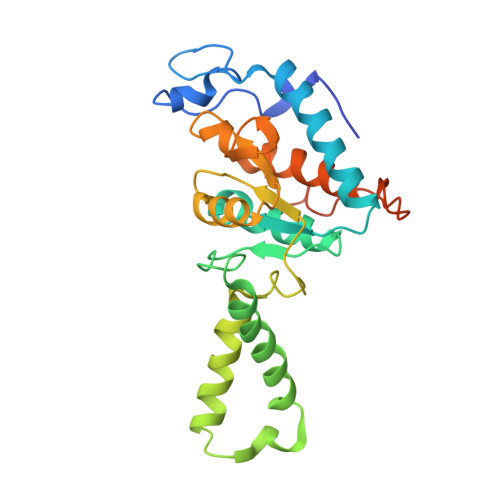
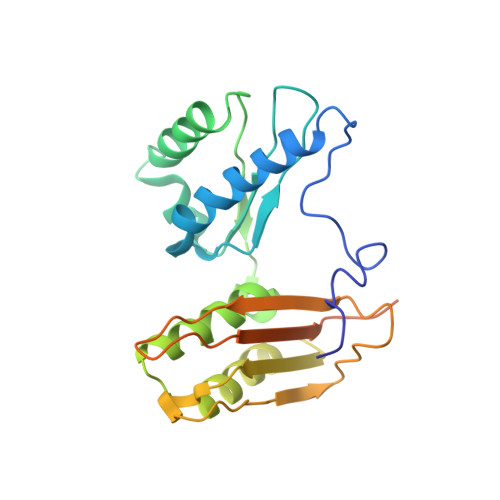
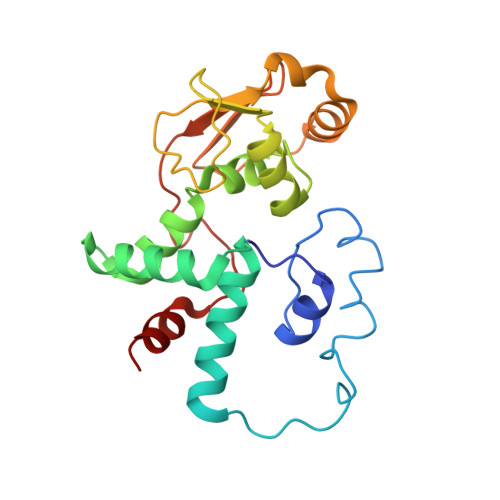
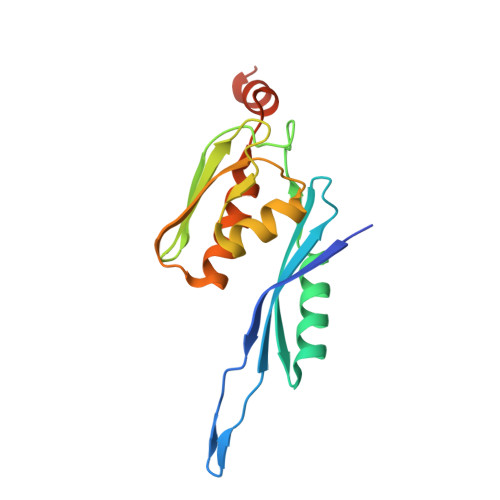
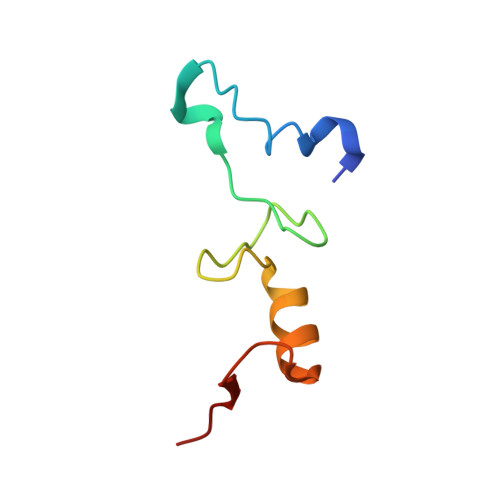
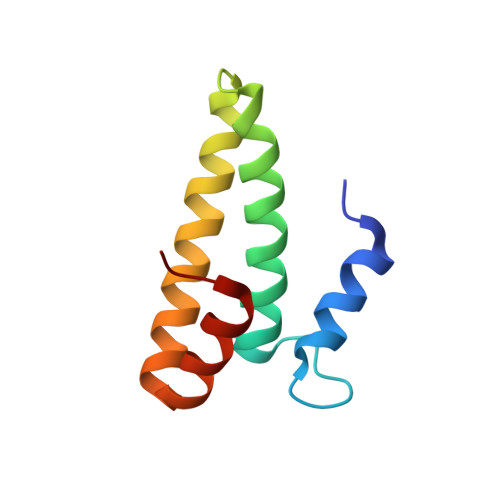
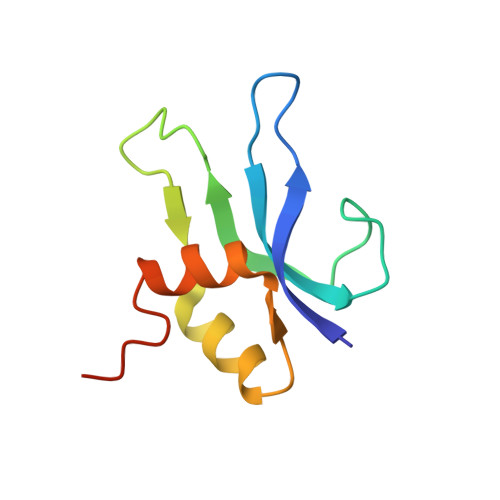
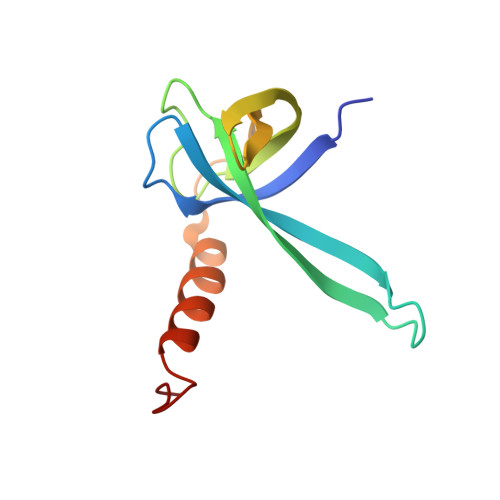
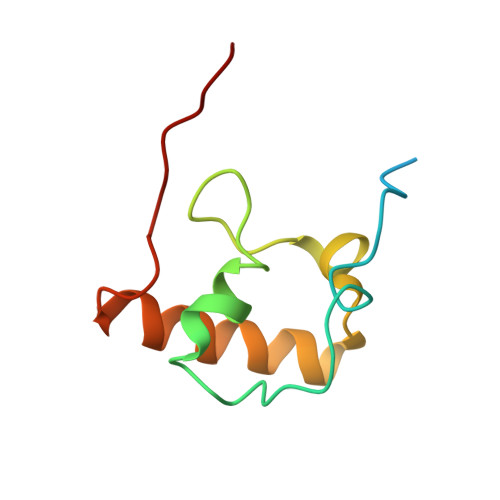
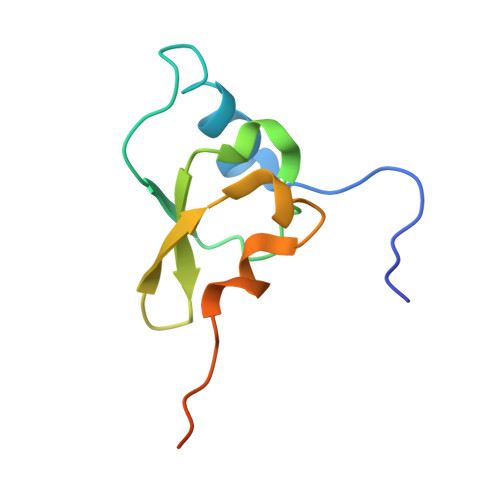
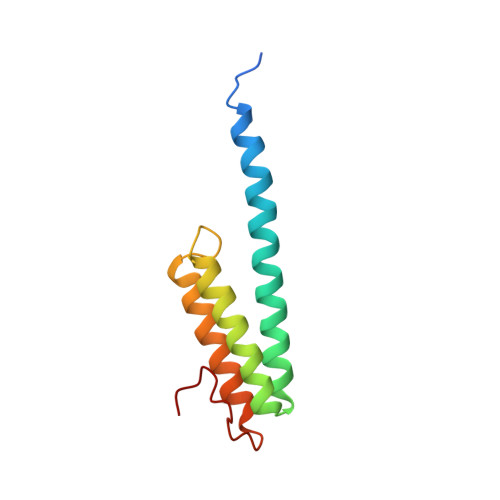
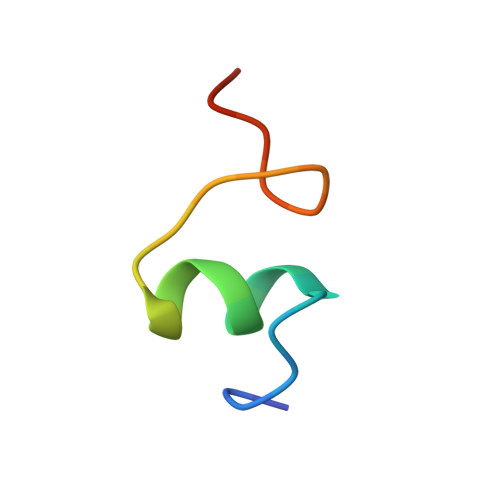
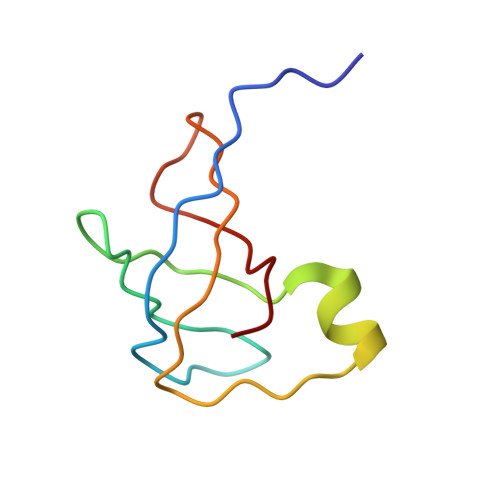
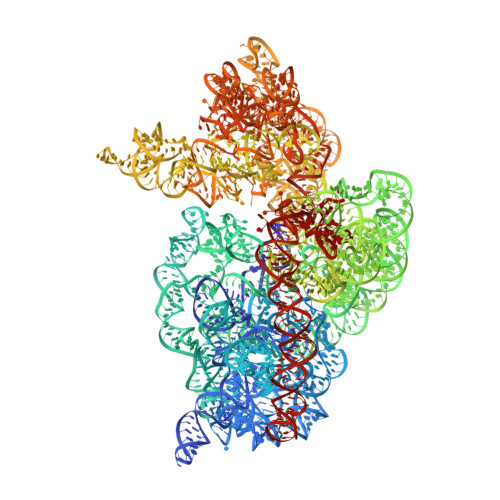
Crystal structure of an initiation factor bound to the 30S ribosomal subunit.
Carter, A.P., Clemons Jr., W.M., Brodersen, D.E., Morgan-Warren, R.J., Hartsch, T., Wimberly, B.T., Ramakrishnan, V.(2001) Science 291: 498-501
- PubMed: 11228145
- DOI: https://doi.org/10.1126/science.1057766
- Primary Citation of Related Structures:
1HR0 - PubMed Abstract:
Initiation of translation at the correct position on messenger RNA is essential for accurate protein synthesis. In prokaryotes, this process requires three initiation factors: IF1, IF2, and IF3. Here we report the crystal structure of a complex of IF1 and the 30S ribosomal subunit. Binding of IF1 occludes the ribosomal A site and flips out the functionally important bases A1492 and A1493 from helix 44 of 16S RNA, burying them in pockets in IF1. The binding of IF1 causes long-range changes in the conformation of H44 and leads to movement of the domains of 30S with respect to each other. The structure explains how localized changes at the ribosomal A site lead to global alterations in the conformation of the 30S subunit.
Organizational Affiliation:
Medical Research Council Laboratory of Molecular Biology, Hills Road, Cambridge CB2 2QH, UK.