Human argininosuccinate lyase: a structural basis for intragenic complementation.
Turner, M.A., Simpson, A., McInnes, R.R., Howell, P.L.(1997) Proc Natl Acad Sci U S A 94: 9063-9068
- PubMed: 9256435
- DOI: https://doi.org/10.1073/pnas.94.17.9063
- Primary Citation of Related Structures:
1AOS - PubMed Abstract:
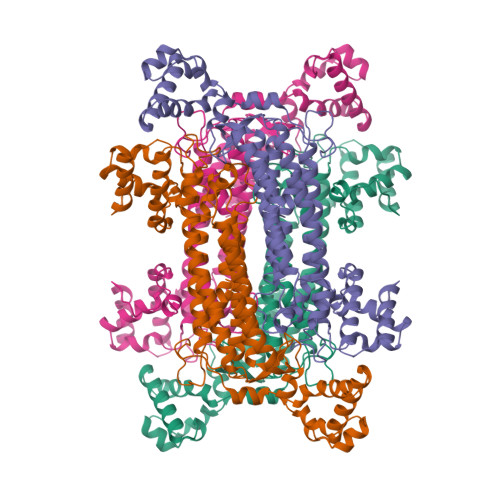
Intragenic complementation has been observed at the argininosuccinate lyase (ASL) locus. Intragenic complementation is a phenomenon that occurs when a multimeric protein is formed from subunits produced by different mutant alleles of a gene. The resulting hybrid protein exhibits enzymatic activity that is greater than that found in the oligomeric proteins produced by each mutant allele alone. The mutations involved in the most successful complementation event observed in ASL deficiency were found to be an aspartate to glycine mutation at codon 87 of one allele (D87G) coupled with a glutamine to arginine mutation at codon 286 of the other (Q286R). To understand the structural basis of the Q286R:D87G intragenic complementation event at the ASL locus, we have determined the x-ray crystal structure of recombinant human ASL at 4. 0 A resolution. The structure has been refined to an R factor of 18. 8%. Two monomers related by a noncrystallographic 2-fold axis comprise the asymmetric unit, and a crystallographic 2-fold axis of space group P3121 completes the tetramer. Each of the four active sites is composed of residues from three monomers. Structural mapping of the Q286R and D87G mutations indicate that both are near the active site and each is contributed by a different monomer. Thus when mutant monomers combine randomly such that one active site contains both mutations, it is required by molecular symmetry that another active site exists with no mutations. These "native" active sites give rise to the observed partial recovery of enzymatic activity.
Organizational Affiliation:
Division of Biochemistry Research, Research Institute, Hospital for Sick Children, 555 University Avenue, Toronto, Ontario M5G 1X8, Canada.