Crystal structure analysis of amicyanin and apoamicyanin from Paracoccus denitrificans at 2.0 A and 1.8 A resolution.
Durley, R., Chen, L., Lim, L.W., Mathews, F.S., Davidson, V.L.(1993) Protein Sci 2: 739-752
- PubMed: 8495197
- DOI: https://doi.org/10.1002/pro.5560020506
- Primary Citation of Related Structures:
1AAJ, 1AAN - PubMed Abstract:
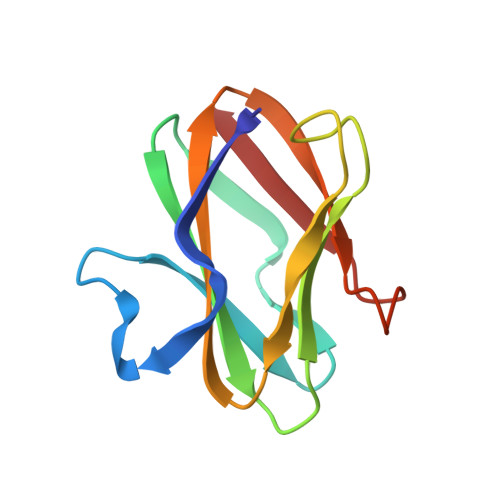
The crystal structure of amicyanin, a cupredoxin isolated from Paracoccus denitrificans, has been determined by molecular replacement. The structure has been refined at 2.0 A resolution using energy-restrained least-squares procedures to a crystallographic residual of 15.7%. The copper-free protein, apoamicyanin, has also been refined to 1.8 A resolution with residual 15.5%. The protein is found to have a beta-sandwich topology with nine beta-strands forming two mixed beta-sheets. The secondary structure is very similar to that observed in the other classes of cupredoxins, such as plastocyanin and azurin. Amicyanin has approximately 20 residues at the N-terminus that have no equivalents in the other proteins; a portion of these residues forms the first beta-strand of the structure. The copper atom is located in a pocket between the beta-sheets and is found to have four coordinating ligands: two histidine nitrogens, one cysteine sulfur, and, at a longer distance, one methionine sulfur. The geometry of the copper coordination is very similar to that in the plant plastocyanins. Three of the four copper ligands are located in the loop between beta-strands eight and nine. This loop is shorter than that in the other cupredoxins, having only two residues each between the cysteine and histidine and the histidine and methionine ligands. The amicyanin and apoamicyanin structures are very similar; in particular, there is little difference in the positions of the coordinating ligands with or without copper. One of the copper ligands, a histidine, lies close to the protein surface and is surrounded on that surface by seven hydrophobic residues. This hydrophobic patch is thought to be important as an electron transfer site.
Organizational Affiliation:
Department of Biochemistry and Molecular Biophysics, Washington University School of Medicine, St. Louis, Missouri 63110.